
HTRF Human Total IDO1 Detection Kit, 500 Assay Points

HTRF Human Total IDO1 Detection Kit, 500 Assay Points




This HTRF kit enables the cell-based quantitative detection of Total IDO1.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
This HTRF kit enables the cell-based quantitative detection of Total IDO1.


HTRF Human Total IDO1 Detection Kit, 500 Assay Points


HTRF Human Total IDO1 Detection Kit, 500 Assay Points


Product information
Overview
The Total IDO1 cell-based assay provides a convenient and accurate way of detecting Total IDO1 levels within cells.
IDO1 (Indoleamine 2,3 dioxygenase 1) is the most important rate-limiting enzyme of the kynurenine pathway due to its ability to catalyze the degradation of L-Tryptophan (Trp) to "kynurenine" metabolites (Kyn). Trp depletion and Kyn production promote immunosuppression by inhibiting CD8+ T effector cells and NK cells. It also increases the activity of CD4+ Treg cells and MDSCs (myeloid-derived suppressor cells). IDO1 also plays a key role in promoting new blood vessel development (angiogenesis).
IDO1 is overexpressed in different tumor cells and infiltrates immune cells, leading to tumor immune escape and neovascularization.
Although IDO1 inhibitors have been developed to inhibit tryptophan metabolism, these therapies have had limited success in prolonging patient survival. Recent efforts have shifted to TPD (targeted protein degradation) of IDO1 as a potentially more efficacious therapeutic strategy.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Technology |
TR-FRET
|
| Unit Size |
500 assay points
|
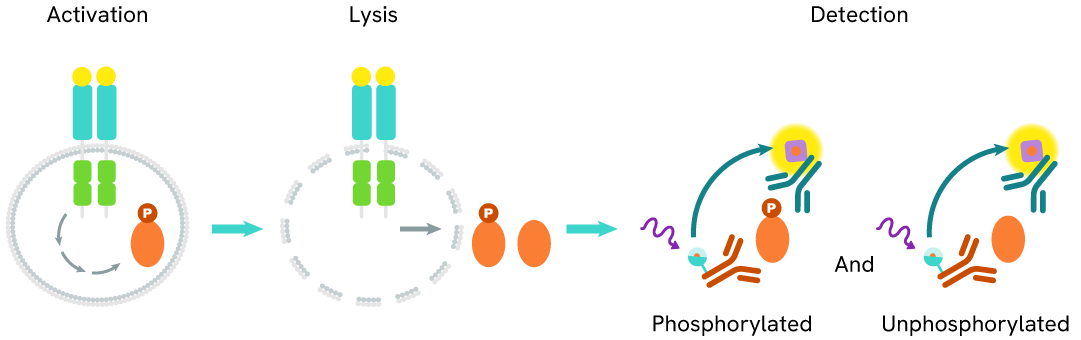
How it works
Total IDO1 assay principle
The Total IDO1 assay quantifies the expression level of IDO1 in a cell lysate. Unlike Western Blot, the assay is entirely plate-based, and does not require gels, electrophoresis, or transfer. The Total IDO1 assay uses two labeled antibodies, one coupled to a donor fluorophore and the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of IDO1 in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor, and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the protein’s expression under a no-wash assay format.

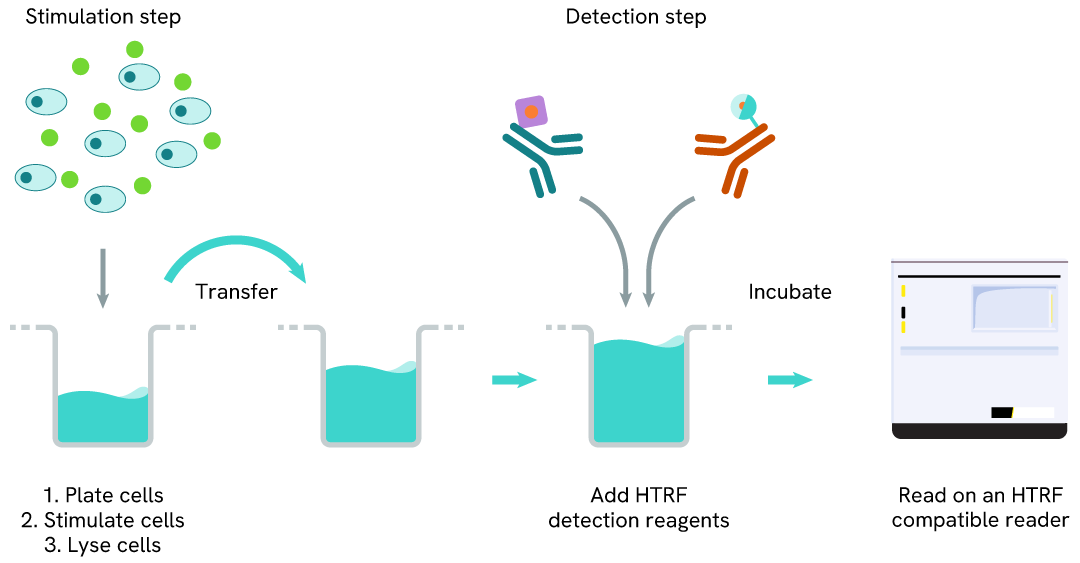
Total IDO1 two-plate assay protocol
The 2-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of the HTRF Total IDO1 detection reagents. This protocol enables the cells' viability and confluence to be monitored.
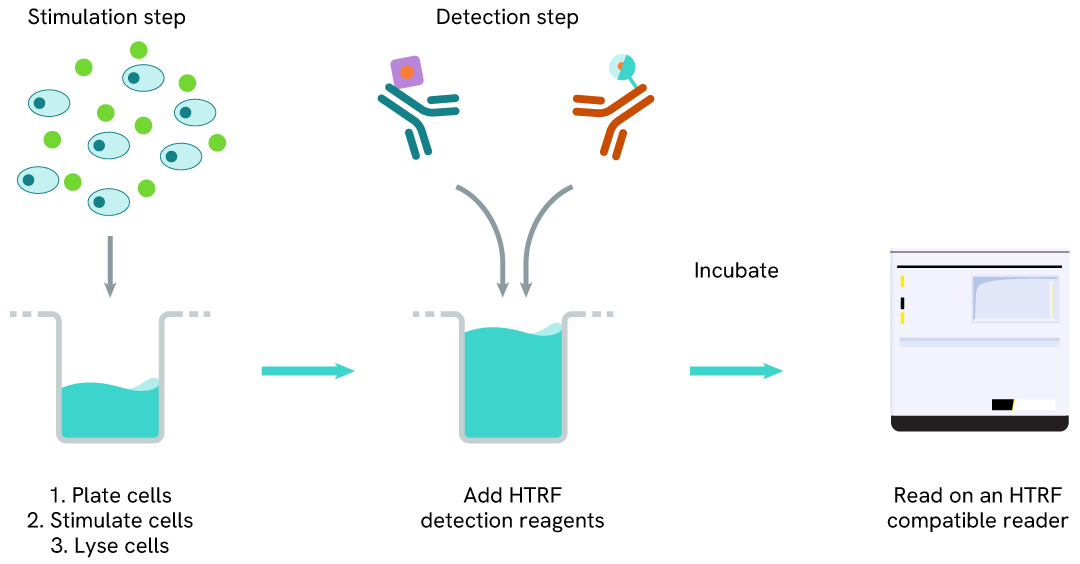
Total IDO1 one-plate assay protocol
Detection of Total IDO1 with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS-designed protocol enables miniaturization while maintaining robust HTRF quality.
Assay validation
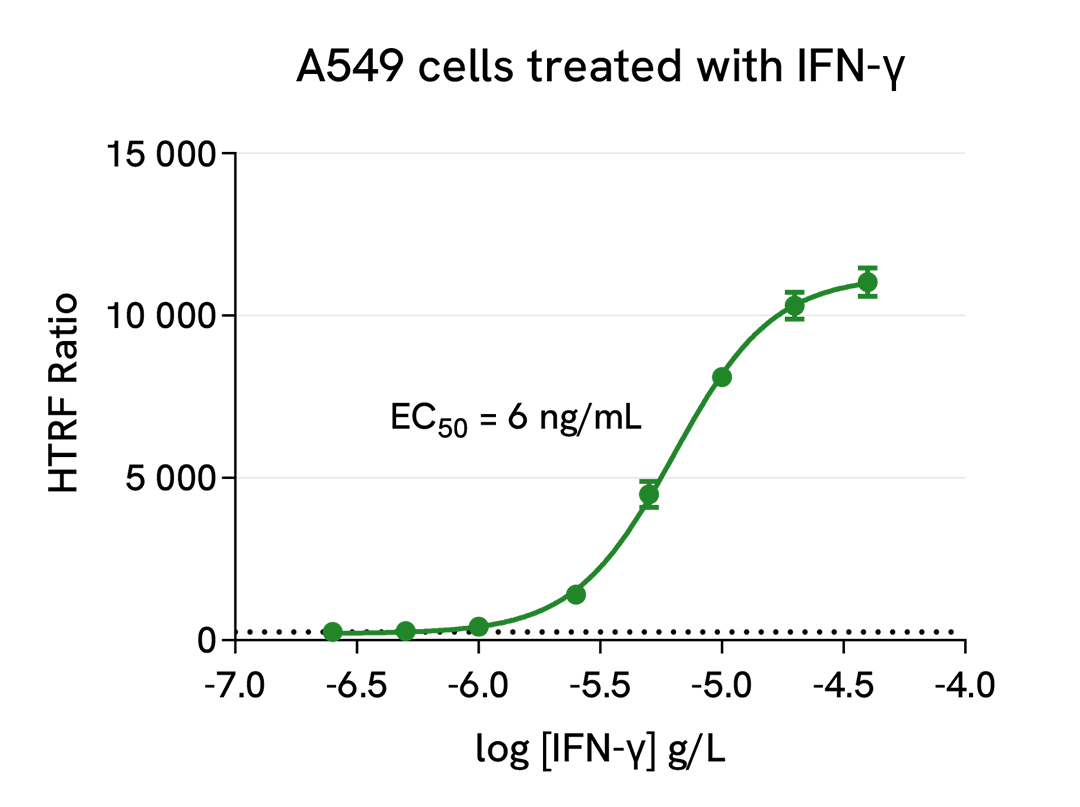
Induction of IDO1 expression by IFN- γ
The experiment was carried out as described in the kit manual for the two-plate assay protocol with adherent cells. A549 cells were plated in a 96-well culture plate (25,000 cells/well in complete culture medium) and cultured overnight at 37°C, 5% CO2. The next day, the cells were treated with increasing doses of recombinant human IFN-γ for 20 hours. The cells were then lysed with 50 µL of supplemented lysis buffer #1, and 16 µL of lysate were transferred into a low volume white microplate (ProxiPlate-384 Plus, Cat # 6008280/9) before the addition of 4 µL of the HTRF Total IDO1 detection antibodies. After a 3h plate incubation at RT, the HTRF signal was recorded on an EnVision Nexus reader (flash lamp mode).
As expected, the IDO1 enzyme was not detected in the human lung cancer cell line A549 in absence of inflammatory stimulus (the dotted line corresponds to the signal of the negative control). After 20h of treatment, the pro-inflammatory cytokine IFN-γ induced a dose-dependent increase in IDO1 expression, with an EC50 value of 6 ng/mL.
Characterization of IDO1 PROTAC degraders
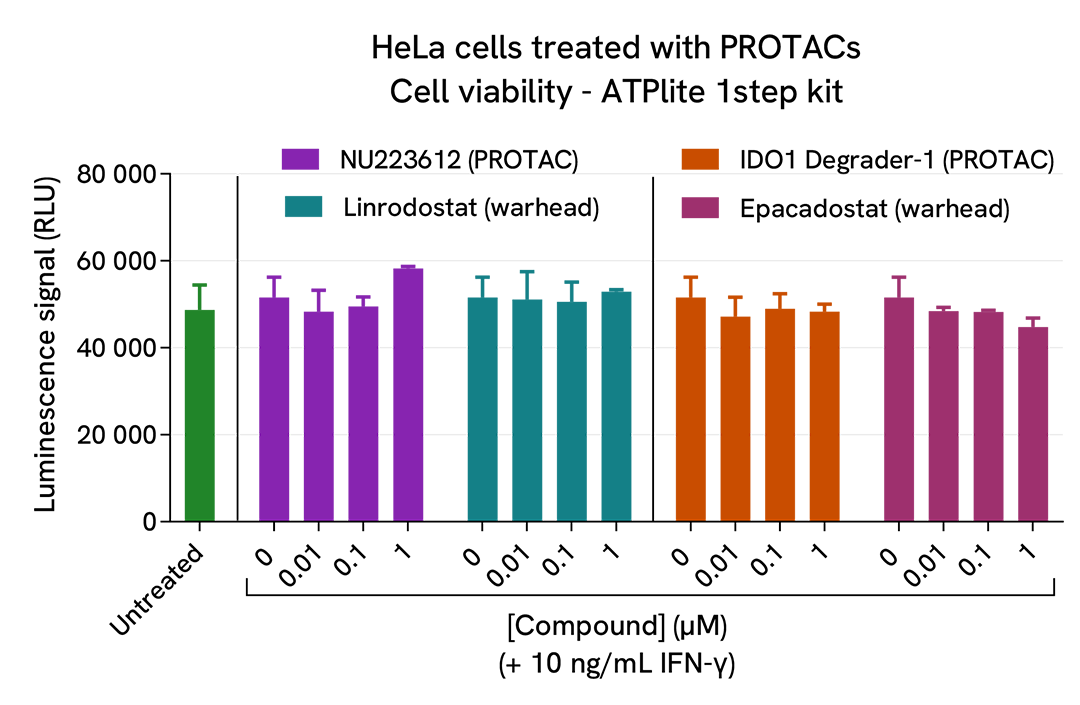
The experiments were carried out according to the two-plate assay protocol for adherent cells. HeLa cells were plated in a 96-well culture plate (25,000 cells/well in complete culture medium) and cultured overnight at 37°C, 5% CO2. The next day, the cells were pre-treated for 7 hours with recombinant human IFN-γ at 10 ng/mL (~ EC50), and then treated for an additional 18h incubation with 3 doses of IDO1 PROTAC degraders (NU223612 or IDO1 Degrader-1), as well as with the respective warheads (Linrodostat or Epacadostat) used as negative controls. At the end of the treatment, the cells were lysed with 50 µL of supplemented lysis buffer #1 and 16 µL of lysate were used to detectTotal IDO1 using the HTRF kit as described in the previous section. Cell viability was also assessed by transferring 5 µL of the same lysate into an HTRF 96-well low volume white plate (Cat # 66PL96005/025/100), followed by the addition of 25 µL of ATPlite 1step reagent (ATPlite 1step Luminescence Assay System, Cat # 6016736/1/9). The luminescence signal was measured on an EnVision Nexus reader after a 10-min incubation in the dark.
As previously observed with A549 cells, the IDO1 enzyme was not detected in the human cervical cancer cell line HeLa in the absence of IFN-γ (green bar; the dotted line corresponds to the signal of the negative control), whereas its expression was well induced by IFN-γ. Both PROTACs dose-dependently degraded IFN-γ-induced IDO1 protein (purple & orange bars), while the respective warheads did not induce any signal decrease (blue & pink bars). In this experiment, the highest IDO1 degradation rate (~50%) was obtained with 1 µM of each PROTAC. These results were further supported by the ATPlite viability assay, where no cytotoxic effects of the different compounds were observed.
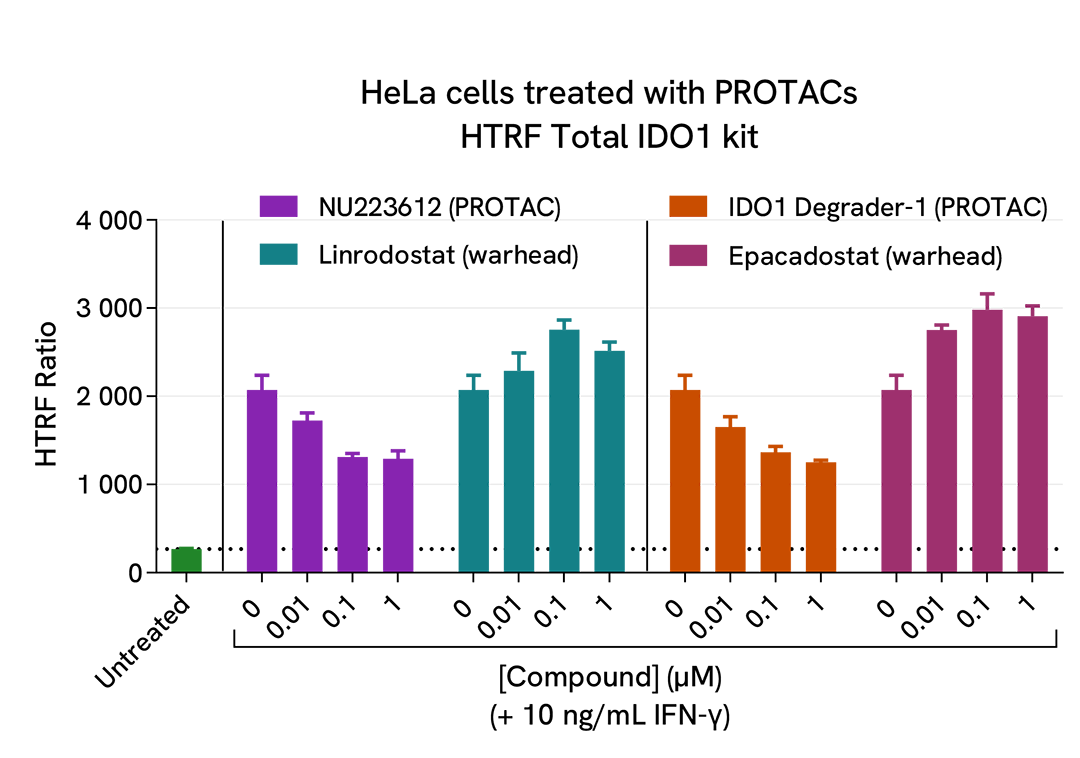
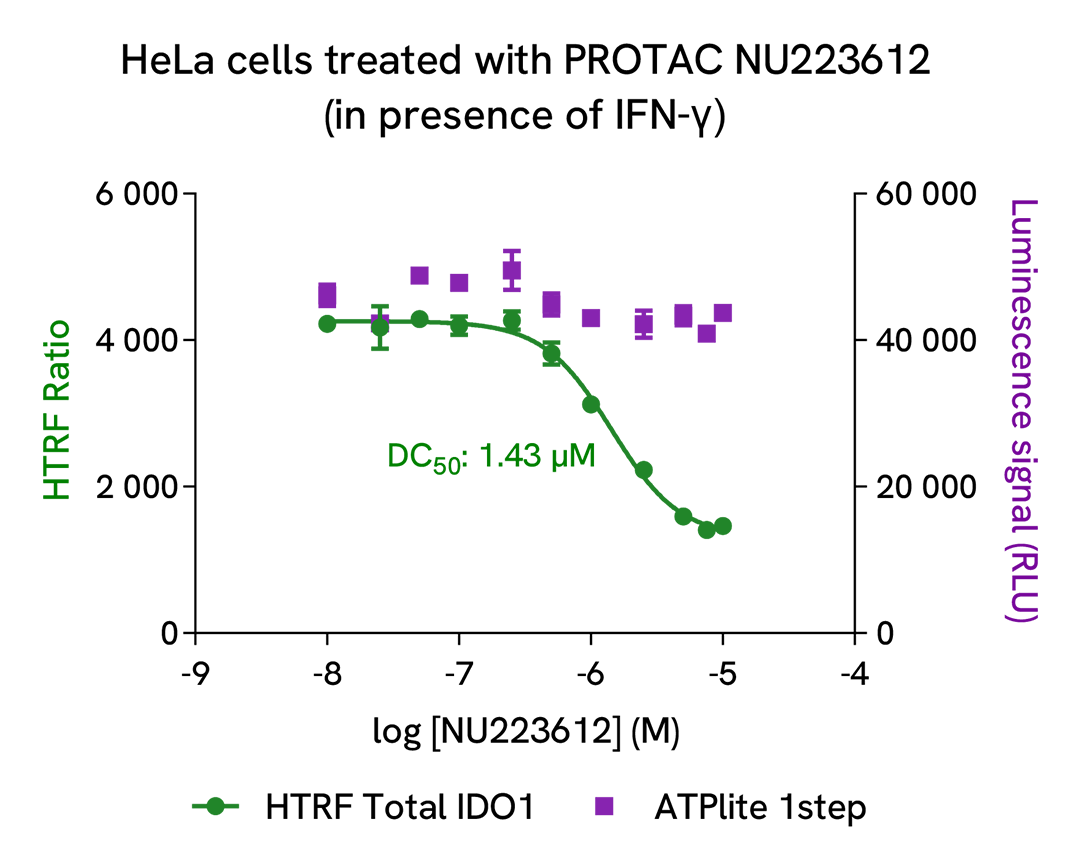
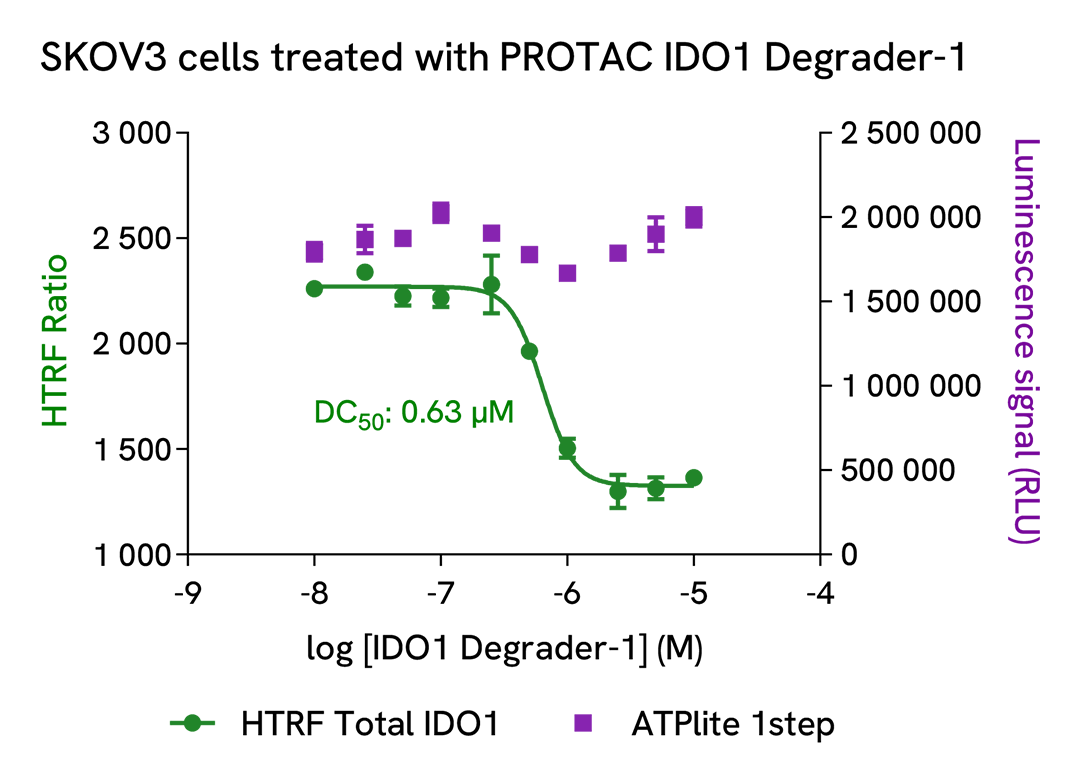
Dose-response experiments with IDO1 PROTACs on HeLa and SKOV3 cell lines
The experiments were conducted using the two-plate assay protocol for adherent cells. HeLa and SKOV3 cells were plated in 96-well culture plates (25,000 and 12,500 cells/well respectively, in complete culture medium) and cultured overnight at 37°C, 5% CO2. HeLa cells were incubated with increasing doses of PROTAC NU223612 (Revvity, in-house synthesis) in presence of 5 ng/mL of recombinant human IFN-γ. SKOV3 cells were treated with various concentrations of PROTAC IDO1 Degrader-1 (MedChemExpress, #HY-131911). After a 20h incubation, the cells were lysed with 50 µL of supplemented lysis buffer #1, and the HTRF Total IDO1 and ATPlite 1step assays were carried out as detailed in the previous sections.
Treatment of HeLa cells with PROTAC NU223612 promoted a dose-dependent degradation of IFN-γ induced IDO1 without affecting cell viability. For this experiment, the DC50 value was 1.43 µM and the highest degradation rate (~70%) was obtained with 7.5 µM of PROTAC. PROTAC IDO1 Degrader-1 dose-dependently degraded constitutive IDO1 protein in SKOV3 cells with no cytotoxic effect. The DC50 value obtained in this experiment was 0.63 µM, and the highest degradation rate (~50%) was obtained with 2.5 µM of degrader.
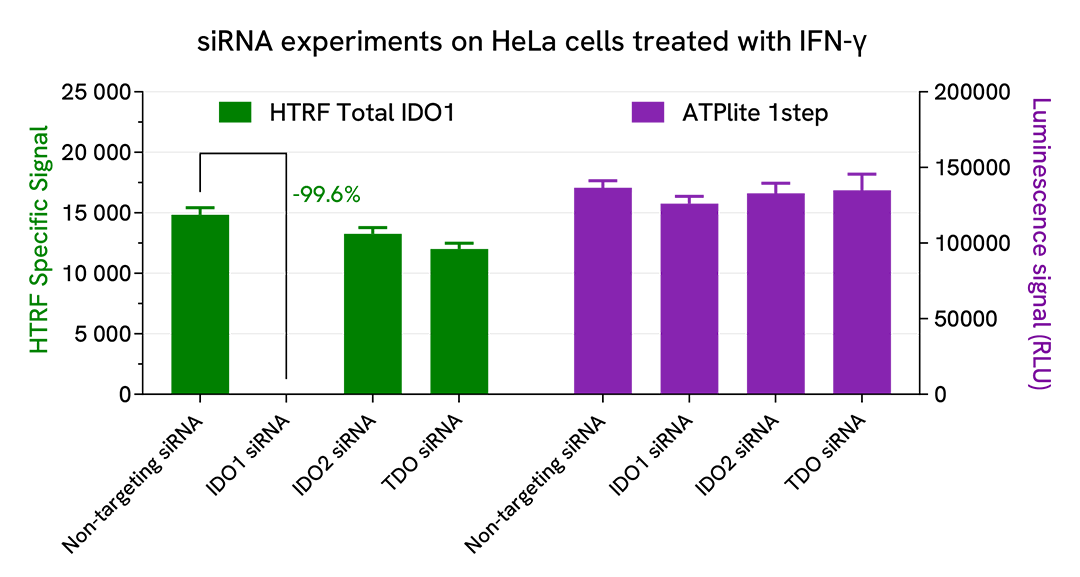
Validation of Total IDO1 assay specificity
HeLa cells were plated in a 96-well culture plate (25,000 cells/well in complete culture medium) and cultured overnight at 37°C, 5% CO2. The next day, the cells were transfected with ON-TARGETplus SMARTPool siRNAs (Horizon Discovery/Revvity) targeting human IDO1 or the related enzymes IDO2 and TDO, as well as with a non-targeting siRNA used as negative control. After a 24h incubation, the medium was renewed and supplemented with 10 ng/mL of recombinant human IFN-γ for an additional 24h incubation. The cells were then lysed with 50 µL of supplemented lysis buffer #1. Total IDO1 was detected using the HTRF kit, and cell viability was measured using the ATPlite 1step luminescence kit, as described in the previous sections.
The siRNA experiments demonstrated that the HTRF detection antibodies specifically measure IDO1 and do not recognize the other related enzymes. It was found that treatment with the IDO1 siRNA induced almost complete HTRF signal loss, while the knockdown of IDO2 and TDO genes did not lead to any significant signal modulation in this assay. The ATPlite luminescence signal was unchanged in presence of siRNAs, demonstrating that the HTRF signal decrease was unrelated to cytotoxic effects.
Assessment of IDO1 expression in human cancer cell lines
The experiments were carried out using the two-plate assay protocol for adherent cells. The human cancer cell lines SKOV3 (ovarian cancer), HeLa (cervical cancer), and A549 (lung cancer) were plated in 96-well culture plates (50,000 cells/well in complete culture medium) and cultured overnight at 37°C, 5% CO2. The next day, the SKOV3 cells were lysed with 50 µL of supplemented lysis buffer #1, whereras the HeLa and A549 cells were treated with 50 ng/mL of recombinant human IFN-γ for 20h before lysis. Total IDO1 levels were detected using the HTRF kit as described in the first section.
The HTRF Total IDO1 assay efficiently detected basal or IFN-γ-induced IDO1 expression in the various human cancer cell lines. As expected, the SKOV3 cell line expressed a high level of constitutive IDO1 protein, while its expression had to be induced by an inflammatory stimulus in HeLa and A549 cells (no detection of IDO1 in untreated cells, as shown in the previous sections).
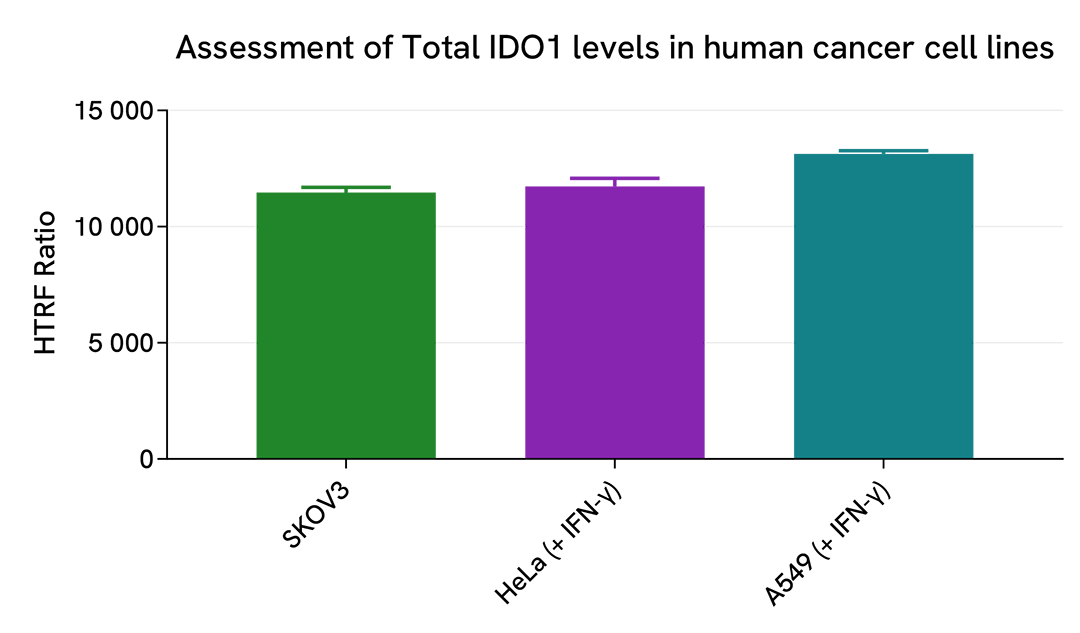
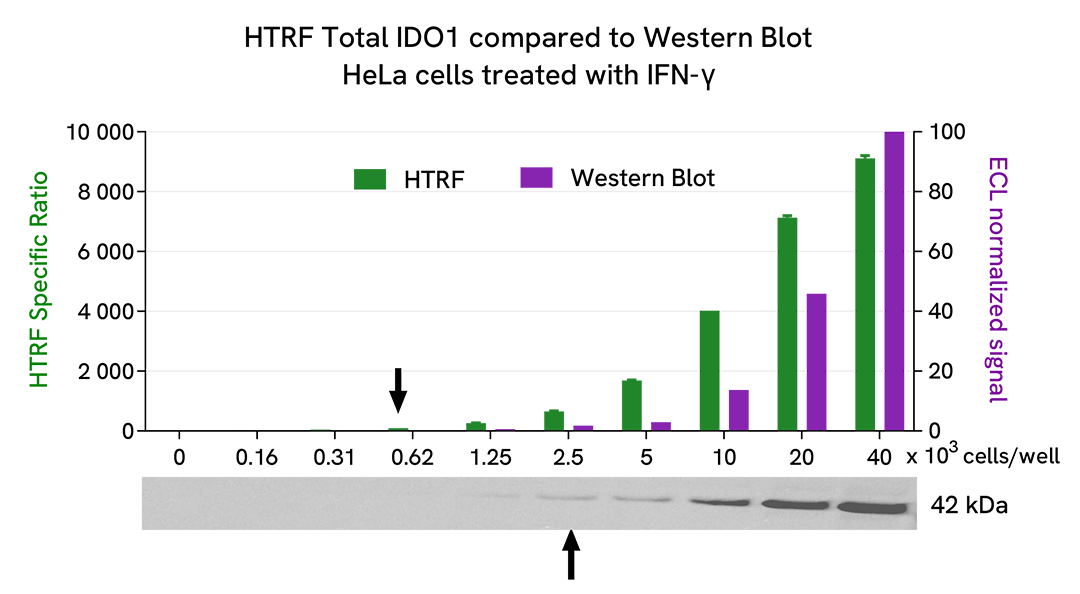
HTRF Total IDO1 assay compared to Western Blot
HeLa cells were seeded in a T175 flask and cultured at 37°C, 5% CO2 in complete culture medium until ~ 80% confluency was reached. The cells were then treated with 50 ng/mL of recombinant human IFN-γ for 20 hours and lysed with 3 mL of supplemented lysis buffer #1. Serial dilutions of the lysate were performed using the supplemented lysis buffer. To detect Total IDO1, 16 µL of each dilution were transferred into a low volume white microplate (ProxiPlate-384 Plus, Cat # 6008280/9), and 4 µL of HTRF Total IDO1 detection reagents were added. Equal amounts of lysates were loaded on a gel for a side-by-side comparison with Western Blot.
Using the HTRF Total IDO1 assay, 625 cells/well were enough to detect a significant signal, while 2,500 cells were needed to obtain a minimal chemiluminescent signal using Western Blot. Therefore, in these conditions the HTRF Total IDO1 assay was 4 times more sensitive than the Western Blot technique.
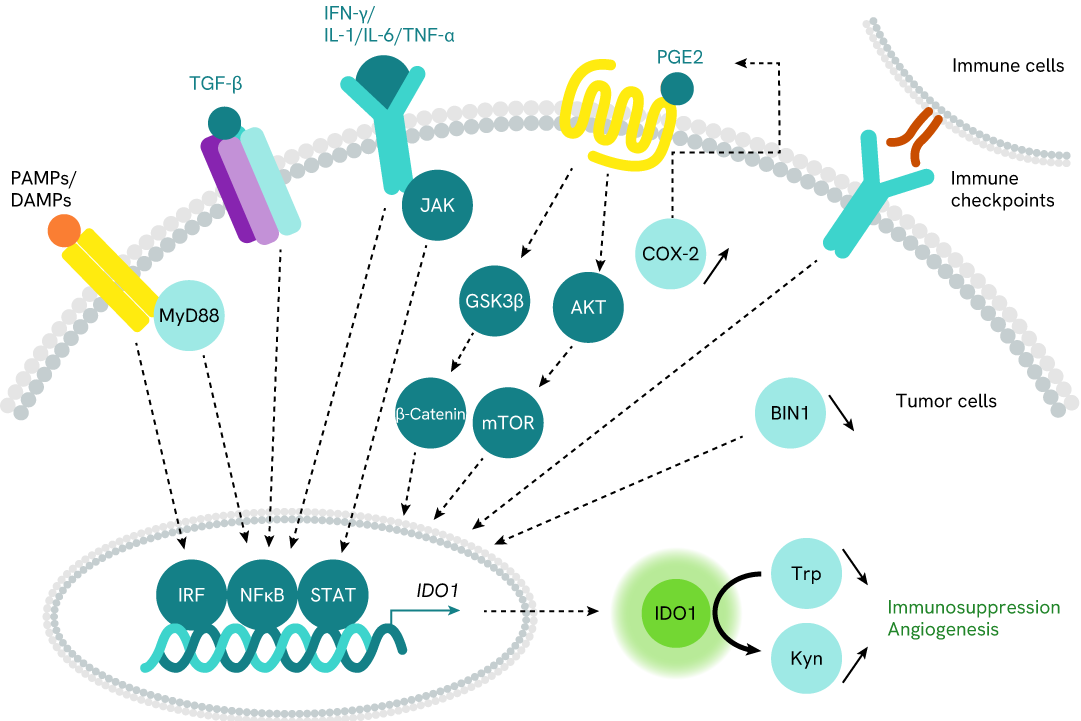
Simplified pathway
IDO1 signaling pathway
The intracellular protein IDO1 (Indoleamine 2,3 dioxygenase 1) is the most important rate-limiting enzyme for the kynurenine pathway, as it catalyzes the degradation of the essential amino acid L-Tryptophan (Trp) to "kynurenine" metabolites (Kyn). Trp depletion and Kyn production promote immunosuppression by inhibiting CD8+ T effector cells and NK cells, as well as by increasing the activity of CD4+ Treg cells and MDSCs (Myeloid-Derived Suppressor Cells). IDO1 also acts as a key node at the regulatory interface between IFN-γ and IL-6 to promote new blood vessel development (angiogenesis).
In most normal cells, IDO1 is not or is only weakly expressed in basal conditions, and is induced by inflammatory stimuli. The cytokine IFN-γ is the most important inducer. Other inflammatory molecules have also been described, such as the cytokines IL-1, IL-6 & TNF-α, PAMPs/DAMPs (TLR pathway), TGF-β, and immune checkpoints (including PD-1 and CTLA-4).
IDO1 is overexpressed in different tumor cells (and infiltrating immune cells), leading to tumor immune escape and neovascularization. The reason for high constitutive expression of IDO1 in malignant cells is either the loss of the cancer-suppression gene BIN1 (Bridging Integrator 1) that enhances IDO1 expression depending on STAT1 and NF-κB, or the overexpression of COX-2 (Cyclooxygenase-2) that activates the PGE2 (Prostaglandin E2) pathway.
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
Your guide for improving cell signaling assay performance
This complete Revvity guide provides you with all the tools you need to...
SDS, COAs, Manuals and more
Are you looking for technical documents related to the product? We have categorized them in dedicated sections below. Explore now.
- LanguageEnglishCountryUnited States
- LanguageFrenchCountryFrance
- LanguageGermanCountryGermany
- Lot Number01ALot DateApril 3, 2026
- Resource TypeManualLanguageEnglishCountry-


Recently Viewed

How can we help you?
We are here to answer your questions.








































