

HTRF Human MMP1 Detection Kit, 10,000 Assay Points
HTRF Human MMP1 Detection Kit, 10,000 Assay Points




The HTRF Human MMP1 Detection Kit enables the specific and detection and quantification of MMP1 in human cell culture supernatants.
| Feature | Specification |
|---|---|
| Application | Protein Quantification |
| Sample Volume | 16 µL |
The HTRF Human MMP1 Detection Kit enables the specific and detection and quantification of MMP1 in human cell culture supernatants.


HTRF Human MMP1 Detection Kit, 10,000 Assay Points


HTRF Human MMP1 Detection Kit, 10,000 Assay Points


Product information
Overview
Matrix metalloproteinase (MMP) 1, also known as interstitial collagenase, is an endopeptidase secreted as an inactive proenzyme that is then activated by proteolytic cleavage. MMP1 plays a significant role in tissue repair and remodeling by degrading structural components of the extracellular matrix (ECM), such as collagens, gelatin, and fibronectin. As such it is closely related to all matrix-dependent disorders, such as fibrotic disease, and some types of cancer that rely on cell adhesion, migration, and invasion.
Specifications
| Application |
Protein Quantification
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Biomarkers
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Unit Size |
10,000 assay points
|
How it works
Human MMP1 assay principle
The Human Matrix MetalloProteinase 1 (MMP1) assay measures MMP1 in cell supernatant. The assay uses two labeled antibodies: one coupled to a donor fluorophore, the other to an acceptor. In presence of Human MMP1 in a sample, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor, and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the capsid present in the sample and therefore provides a means of assessing any changes caused by experimental variables, in a no-wash assay format.

Human MMP1 assay protocol
The Human MMP1 assay protocol, using a 384-well small volume white plate, is described on the right. 16 µL of sample or standard are dispensed directly into the detection plate for detection by HTRF® reagents. The antibodies labeled with HTRF fluorophores are added before an overnight incubation. The assay can be run in up to a 1536-well format by simply resizing each addition volume proportionally.
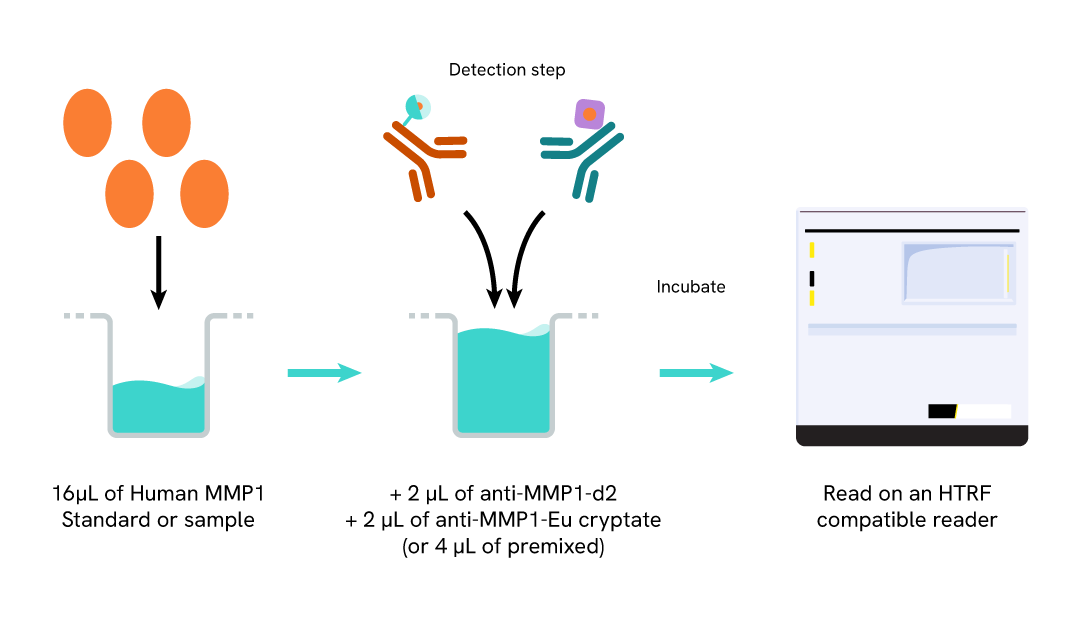
Assay details
Human MMP1 assay details
| Sample size | 16 µL |
|---|---|
| Final assay volume | 20 µL |
| Time to result | Overnight at RT |
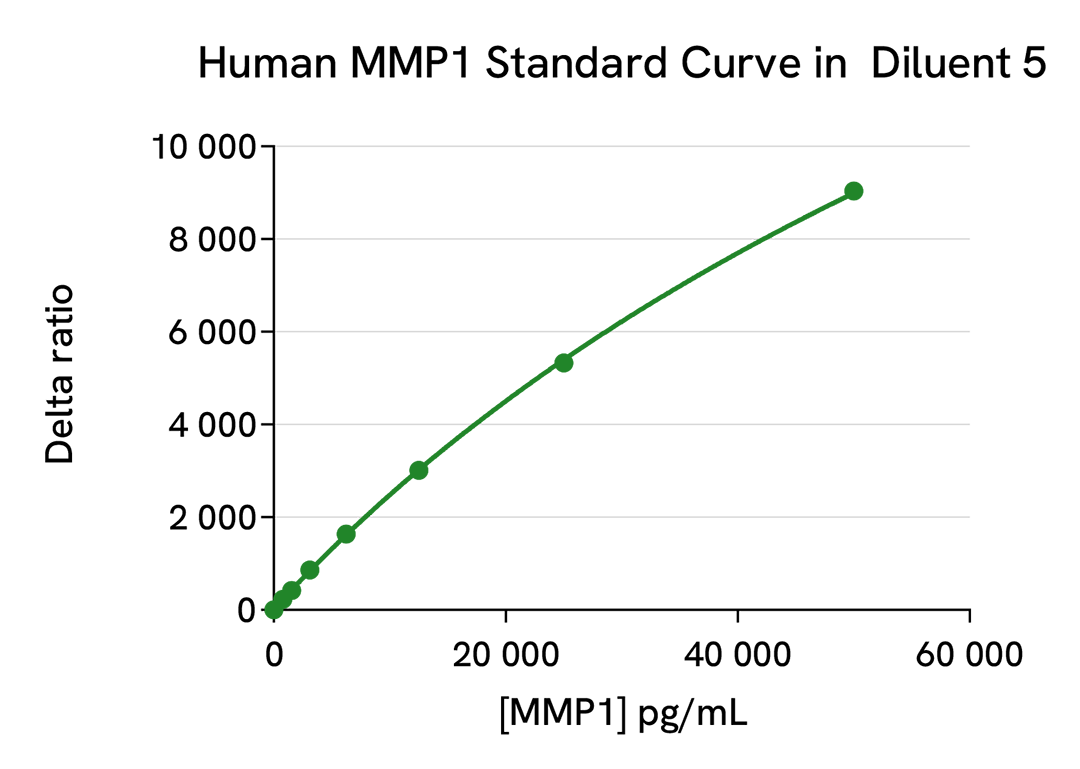
| Detection Limit (LOD) in Diluent | 46.5 pg/mL |
| Dynamic Range | 181 – 50 000 pg/mL |
| Species | Human only |
Analytical performance
Intra-assay
Each of the 3 samples was measured 24 times, and the % CV was calculated for each sample.
Samples were cell culture supernatants from unstimulated MRC-5 cells.
|
Sample |
Mean [Human MMP1] (pg/mL) |
CV |
|---|---|---|
|
1 |
9 830 |
3% |
|
2 |
3 280 |
4% |
|
3 |
1 090 |
5% |
|
|
Mean CV |
4% |
Inter-assay
Each of the samples was measured in 4 independent experiments by 4 different operators, and the % CV was calculated for each sample. Samples were cell culture supernatants from unstimulated MRC-5 cells.
|
Sample |
Mean [Human MMP1] (pg/mL) |
CV |
|---|---|---|
|
1 |
6 710 |
4% |
|
2 |
3 430 |
7% |
|
3 |
1 720 |
10% |
|
|
Mean CV |
7% |
Dilutional linearity
Samples were from primary human lung fibroblast supernatants serially diluted in cell culture medium.
The recovery % obtained from these experiments show the good dilutional linearity of the assay (dilution test acceptance criteria: 85-115%).
|
Dilution Factor |
[MMP1] Expected (pg/mL) |
[MMP1] Measured (pg/mL) |
Dilution Recovery |
|---|---|---|---|
|
Neat |
- |
49 913 |
100% |
|
2 |
24 956 |
26 783 |
107% |
|
4 |
12 478 |
13 819 |
111% |
|
8 |
6 239 |
6 682 |
107% |
|
16 |
3 120 |
3 031 |
97% |
|
|
Mean CV |
|
104% |
Antigen spike and recovery
Four concentrations of recombinant protein (~2815 – 1268 – 546 and 164 pg/mL) were added to 2 dilutions of native sample from primary human lung fibroblast supernatants, and the expected concentrations were compared to those measured in order to compute antigen recoveries (acceptance criteria: 85-115%). The recovery was good after overnight incubation at room temperature. The 100% recovery indicates similar measurements of MMP1 from samples and the kit standard.
|
Sample |
[MMP1] Standard(pg/mL) |
[MMP1]Spiked Sample (pg/mL) |
Expected (pg/mL) |
Measured (pg/mL) |
Recovery |
|---|---|---|---|---|---|
|
1 |
2 815 |
9 700 |
12 516 |
13 435 |
107% |
|
1 564 |
4 379 |
4 652 |
106% |
||
|
2 |
1 268 |
9 700 |
10 969 |
11 600 |
106% |
|
1 564 |
2 832 |
2 999 |
106% |
||
|
3 |
546 |
9 700 |
10 246 |
11 200 |
109% |
|
1 564 |
2 110 |
2 239 |
106% |
||
|
4 |
164 |
9 700 |
9 864 |
10 993 |
111% |
|
1 564 |
1 728 |
1 940 |
112% |
||
|
|
Mean CV |
|
|
|
108% |
Interferences
The possible interference from human Tissue Inhibitors of Metalloproteinases (TIMP-1, TIMP-2, TIMP-3, and TIMP-4) and α2-macroglobulin was investigated. The human MMP1 was kept at a constant concentration (25 000 pg/mL). The binding proteins were titrated into the assay. No significant interference was observed up to 100 000 pg/mL, which was the maximum concentration tested.
|
Tested Protein |
Interferences (%)100 000 pg/mL |
|---|---|
|
TIMP1 |
No |
|
TIMP2 |
No |
|
TIMP3 |
No |
|
TIMP4 |
No |
|
α2-macroglobulin |
-18% |
Cross reactivities
Cross reactivities were assessed using recombinant proteins from the MMP family. Proteins were tested up to 30.000 pg/mL and standard curves were generated for each protein diluted in the kit diluent.
16 µL of sample were transferred into a white detection plate (384 low volume), and 4 µL of a mixture of the HTRF Human MMP1 detection reagents were added. The HTRF signal was recorded after an overnight incubation at room temperature.
Signals were plotted on the assay standard curve to interpolate concentrations. Cross-reactivity was calculated as a mean of 6 tested concentrations. The assay is human specific and mouse MMP1 was not detected by it.
|
Tested Protein |
Cross Reactivity |
|---|---|
|
Human MMP1 (Pro & Active forms) |
100% |
|
Mouse MMP1 |
No |
|
Human MMP2 |
No |
|
Human MMP3 |
No |
|
Human MMP8 |
No |
|
Human MMP9 |
No |
|
Human MMP13 |
No |
Assay validation
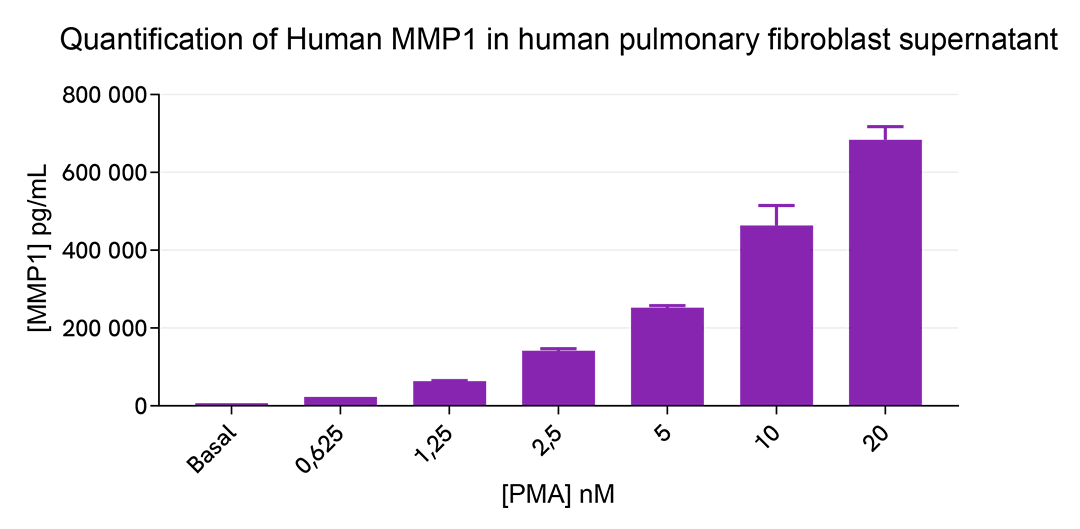
MMP1 upregulation induced by different treatments in human pulmonary fibroblast cell line MRC-5
The human lung fibroblast cell line MRC-5 was plated in a 96-well plate (12,500 cells/well). The day after, cells were treated overnight with 50 µL of increasing concentrations of PMA diluted in FBS-free medium supplemented with 1% BSA. Cell supernatants were collected for the quantitative measurement of secreted MMP1 levels.
16 µL of supernatants (diluted ¼) were then transferred into a white detection plate (384 low volume) and 4 µL of the HTRF Human MMP1 detection reagents were added. The HTRF signal was recorded after an overnight incubation at room temperature.
As expected, PMA treatment led to a dose-dependent increase in the secretion of human MMP1.
The human lung fibroblast cell line MRC-5 was plated in a 96-well plate (12,500 cells/well). The day after, cells were treated overnight with 50 µL of increasing concentrations of human TNF alpha or TGF-β1 diluted in FBS-free medium supplemented with 1% BSA. Cell supernatants were collected for the quantitative measurement of secreted MMP1 levels.
16 µL of supernatants (diluted ¼) were then transferred into a white detection plate (384 low volume) and 4 µL of the HTRF Human MMP1 detection reagents were added. The HTRF signal was recorded after an overnight incubation at room temperature.
As expected, cell treatment for 24h induced an upregulation of the secreted level of human MMP1, particularly after TNF alpha treatment.
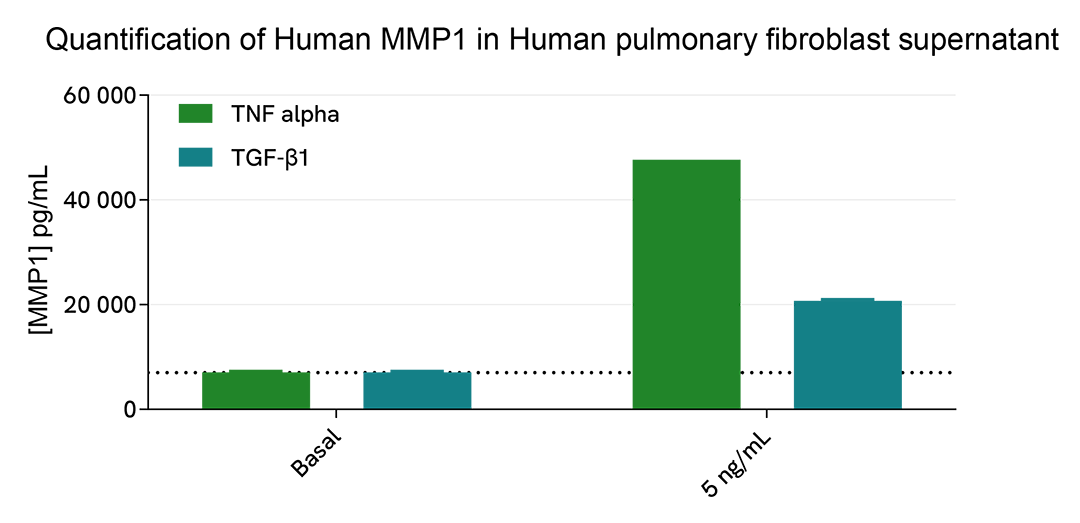
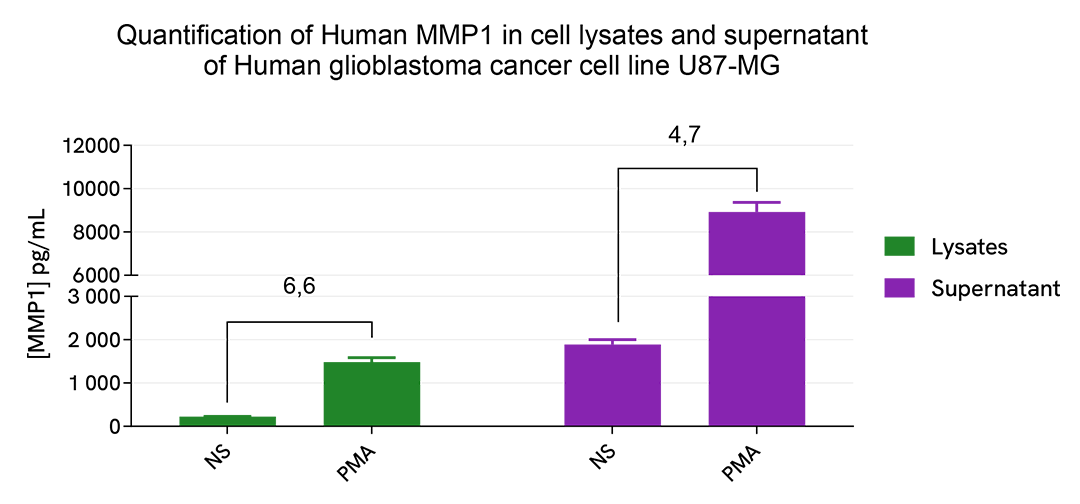
MMP1 upregulation induced by PMA treatment in cell lysates and cell supernatant of human glioblastoma cancer cell line U87-MG
The human glioblastoma cancer cell line U87-MG was plated in a 96-well plate (100,000 cells/well). The day after, cells were treated overnight with 50 µL of PMA (50 ng/mL) diluted in FBS-free medium supplemented with 1% BSA.
Cell supernatants were collected, and cells were lysed with 50 µL of supplemented lysis buffer #3 (4X) for 30 min at room temperature under gentle shaking for the quantitative measurement of MMP1 levels.
16 µL of supernatants or lysates were then transferred into a white detection plate (384 low volume) and 4 µL of the HTRF Human MMP1 detection reagents were added. The HTRF signal was recorded after an overnight incubation at room temperature.
As expected, PMA treatment led to an increase in the intracellular and secreted levels of human MMP1 in this cancer cell line.
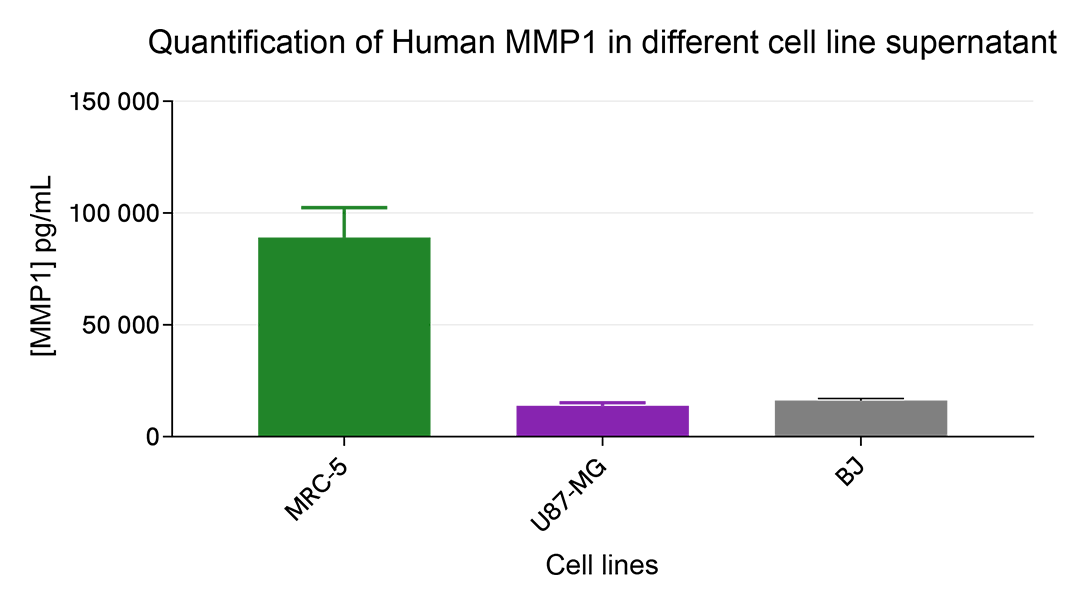
Versatility of Human MMP1 assay in human cell line supernatants
Different human cell lines were seeded in T175 flasks in 25 mL of complete culture medium at 37°C, 5% CO2 up to 80% confluence. Then the supernatant was collected. 16 µL of each cell line supernatant were transferred into a 384 well low volume white microplate and 4 µL of HTRF Human MMP1 detection antibody were added. The HTRF signal was recorded after an overnight incubation. The results reveal a differential expression pattern with a high concentration level in the human pulmonary fibroblast cell line and a lower level in the human glioblastoma cancer cell line (U87-MG) or Human foreskin BJ fibroblasts.
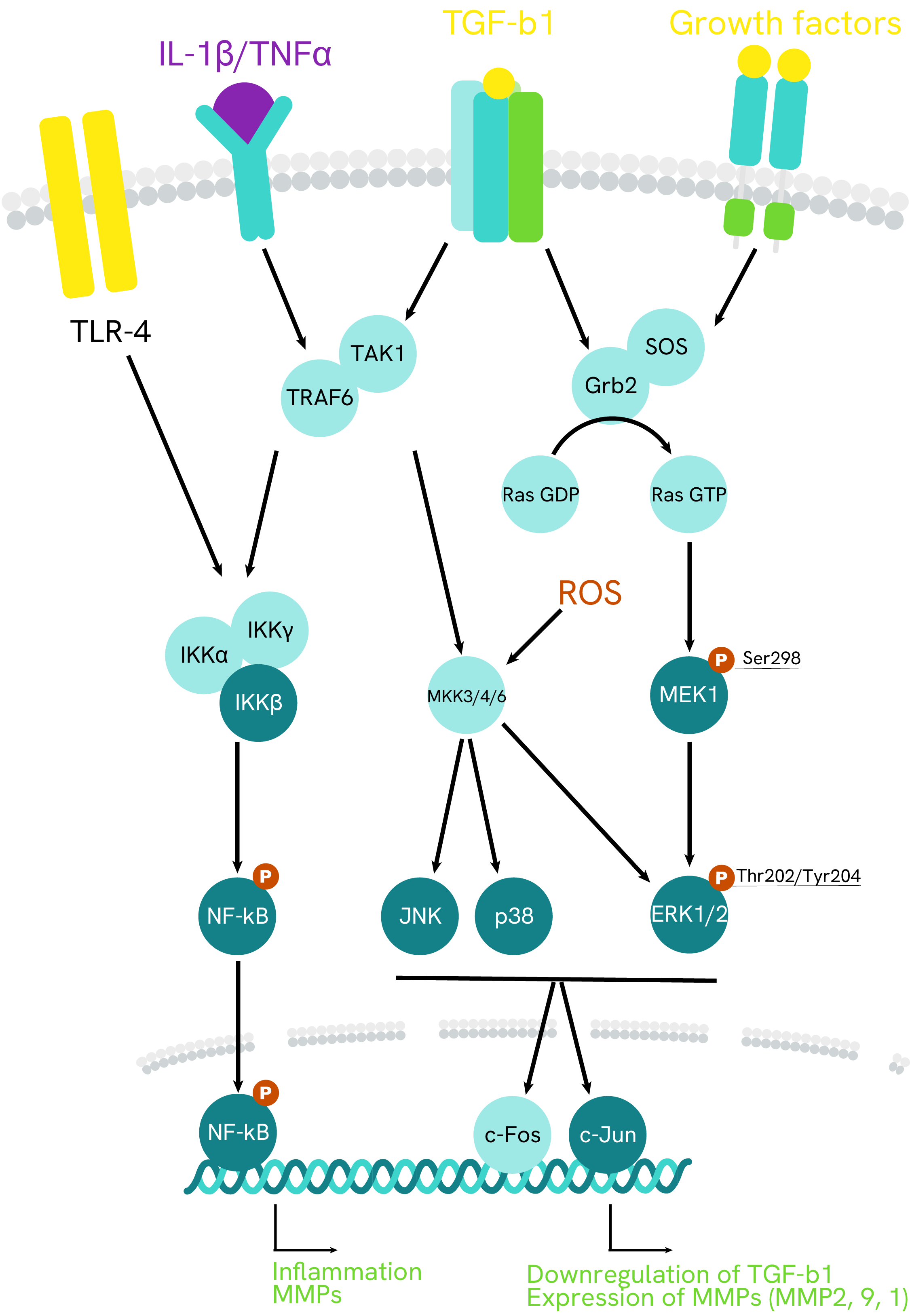
Simplified pathway
MMP1 signaling pathway
Matrix metalloproteinases (MMPs) are a family, 23 members of which have been identified, produced by diverse cell types, including immune cells, epithelial cells, and fibroblasts. MMP functions include degradation of components of the extracellular matrix (ECM) in normal physiological processes, such tissue remodeling, and in disease processes, such as cancer. Specifically, MMP-1 breaks down the interstitial collagens, types I, II, and III.
MMP1, or collagenase-1, plays critical roles in many physiologic processes by degrading these collagen types I, II, and III.
The regulation of MMP1 is complex and multifactorial. All the pathways involved seem to converge toward JNK and its resulting activation of transcription factors c-Jun and c-Fos. Various growth factors and cytoskeleton-related mechanical tension may be involved.
Cytokines are also involved in MMP1 regulation. Pro-inflammatory IL-1β in particular promotes them via the NF-kB pathway or via p38 MAP kinase, particularly in cancer.
MMP1 are secreted or expressed at the membrane as precursors and their activity is regulated by the TIMPs (tissue inhibitors of metalloproteinases) and by themselves, as they activate each other in a complex regulatory network.
Resources
Are you looking for resources, click on the resource type to explore further.
HTRF: unfamiliar territory?
This technical brochure reviews the general principles of HTRF™ and the associated Tag-lite™ technology...
Loading...


How can we help you?
We are here to answer your questions.








































