
HTRF Human Phospho-IRE1 (Ser724) Detection Kit, 500 assay points
HTRF Human Phospho-IRE1 (Ser724) Detection Kit, 500 assay points


This HTRF kit allows for the cell-based quantitative detection of IRE1 when phosphorylated at Ser724.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
This HTRF kit allows for the cell-based quantitative detection of IRE1 when phosphorylated at Ser724.


HTRF Human Phospho-IRE1 (Ser724) Detection Kit, 500 assay points


HTRF Human Phospho-IRE1 (Ser724) Detection Kit, 500 assay points


Product information
Overview
IRE1 (Inositol requiring enzyme 1 alpha) is a serine/threonine kinase acting as one of three branches of the Unfolded Protein Response (UPR) signaling pathway with roles in regulation of ER stress and UPR. IRE1 is encoded by the ERN1 gene and is ubiquitously expressed in mammals. IRE1 is a type I transmembrane protein, exhibiting an ER luminal domain that senses the protein folding status and a catalytic kinase and RNase cytosolic domain. In recent years, IRE1 has emerged as a relevant therapeutic target in various diseases including degenerative disorders, inflammatory and metabolic pathologies, and cancer.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target |
IRE1
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Central Nervous System
Inflammation
Oncology
|
| Unit Size |
500 assay points
|
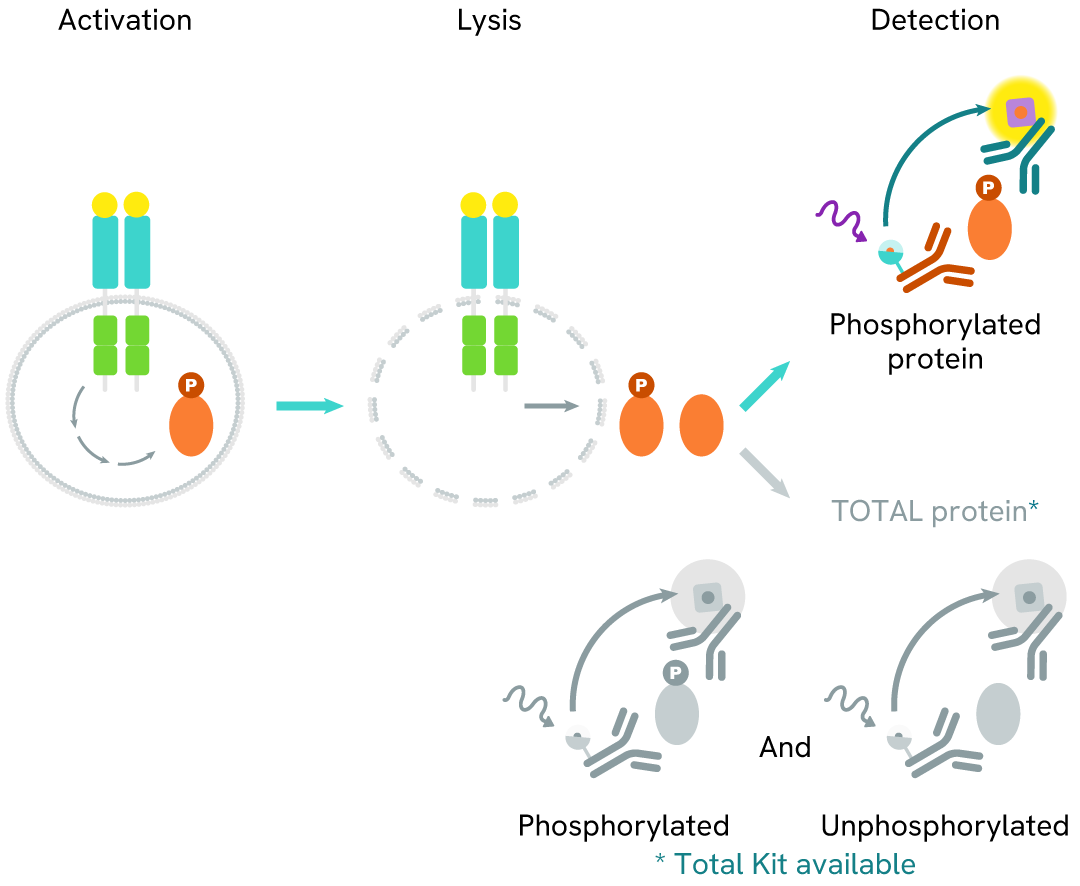
How it works
Phospho-IRE1 (Ser724) assay principle
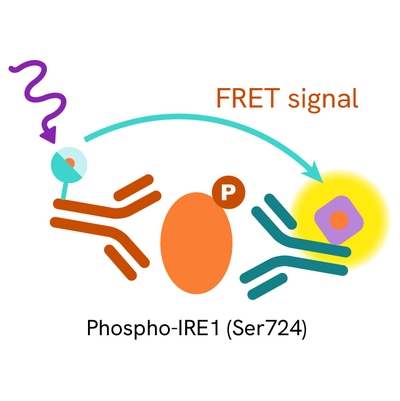
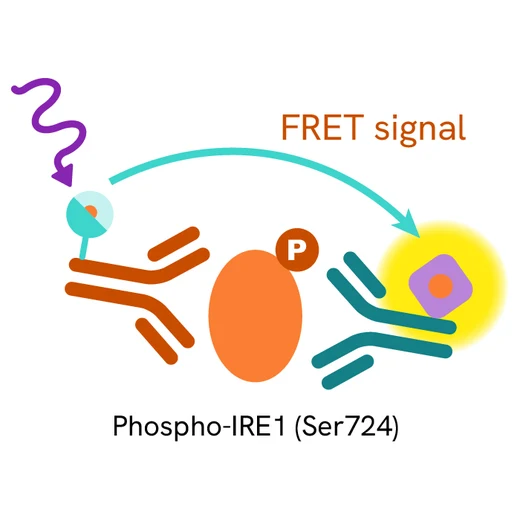
The Phospho-IRE1 (Ser724) assay measures DDR1 when phosphorylated at Ser724. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The assay uses 2 antibodies, one labeled with a donor fluorophore and the other with an acceptor. The first antibody was selected for its specific binding to the phosphorylated motif on the protein, and the second for its ability to recognize the protein independently of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies, and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal.
Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample and provides a means of assessing the protein's phosphorylation state under a no-wash assay format.

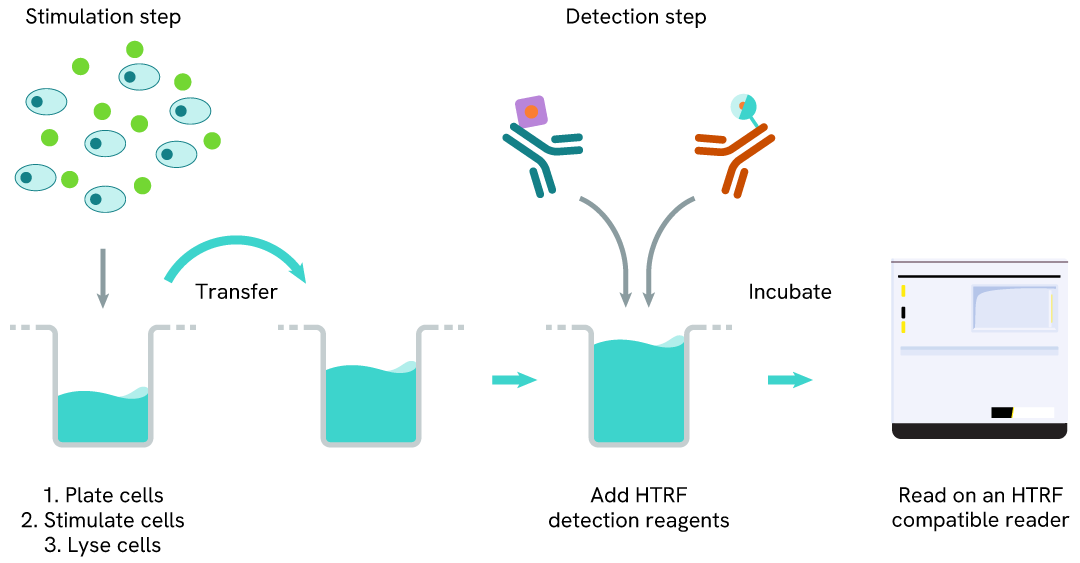
Phospho-IRE1 (Ser724) two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of Phospho-IRE1 (Ser724) HTRF detection reagents. This protocol allows for the cells' viability and confluence to be monitored.
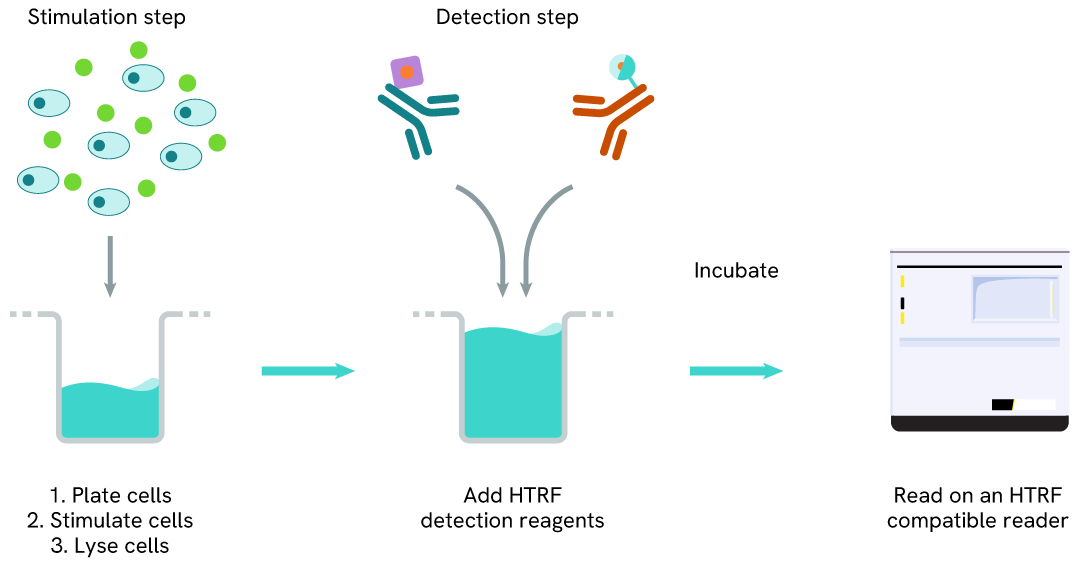
Phospho-IRE1 (Ser724) one-plate assay protocol
Detection of Phosphorylated IRE1 (Ser724) with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol allows miniaturization while maintaining robust HTRF quality.
Assay validation
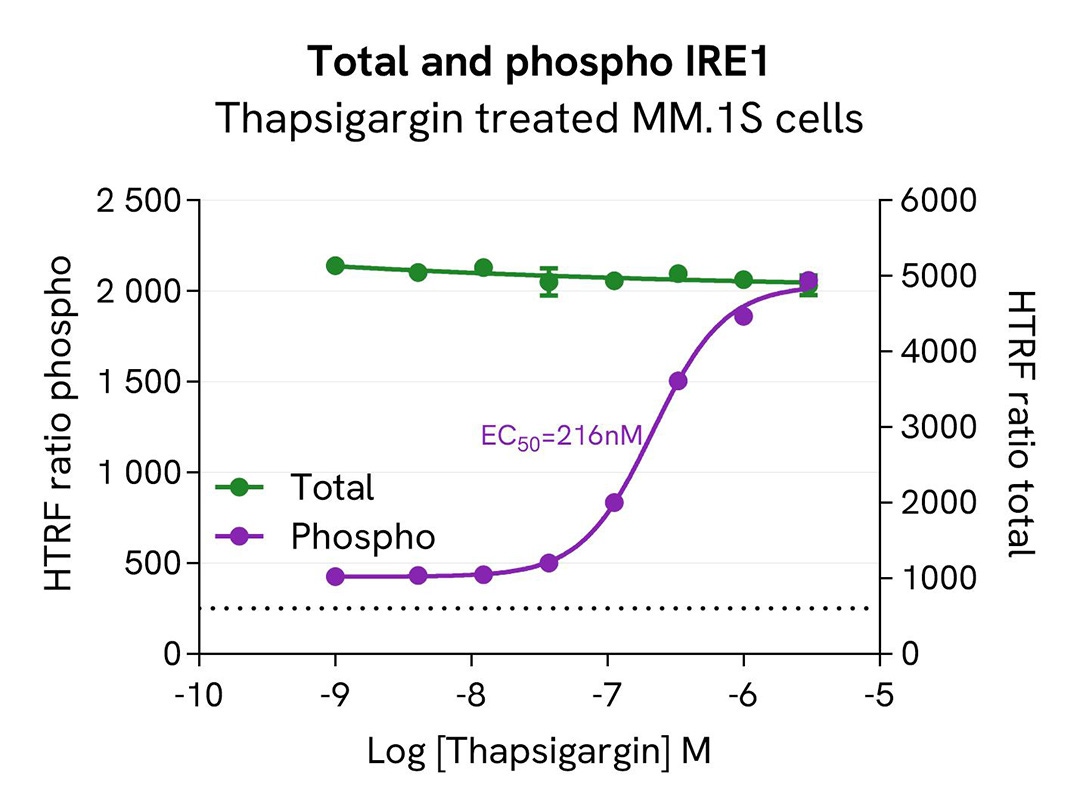
Induction of Phospho-IRE1 (Ser724) in MM.1S cells
MM.1S cells were seeded in a 96-well culture plate (400,000 cells/30µL well) and treated with increasing concentrations of Thapsigargin.
After a 1 hour treatment, cells were lysed with 10 µL of supplemented lysis buffer #1 (4X) for 30 min at RT under gentle shaking. Next, 16 µL of lysate were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF Phospho-IRE1 (S724) or HTRF Total IRE1 detection antibodies were added. HTRF signals were recorded after 4 hours of incubation.
Our results indicate a clear dose-dependent activation of IRE1 phosphorylation at Ser724 upon treatment with Thapsigargin, while the total IRE1 protein expression level remained constant.
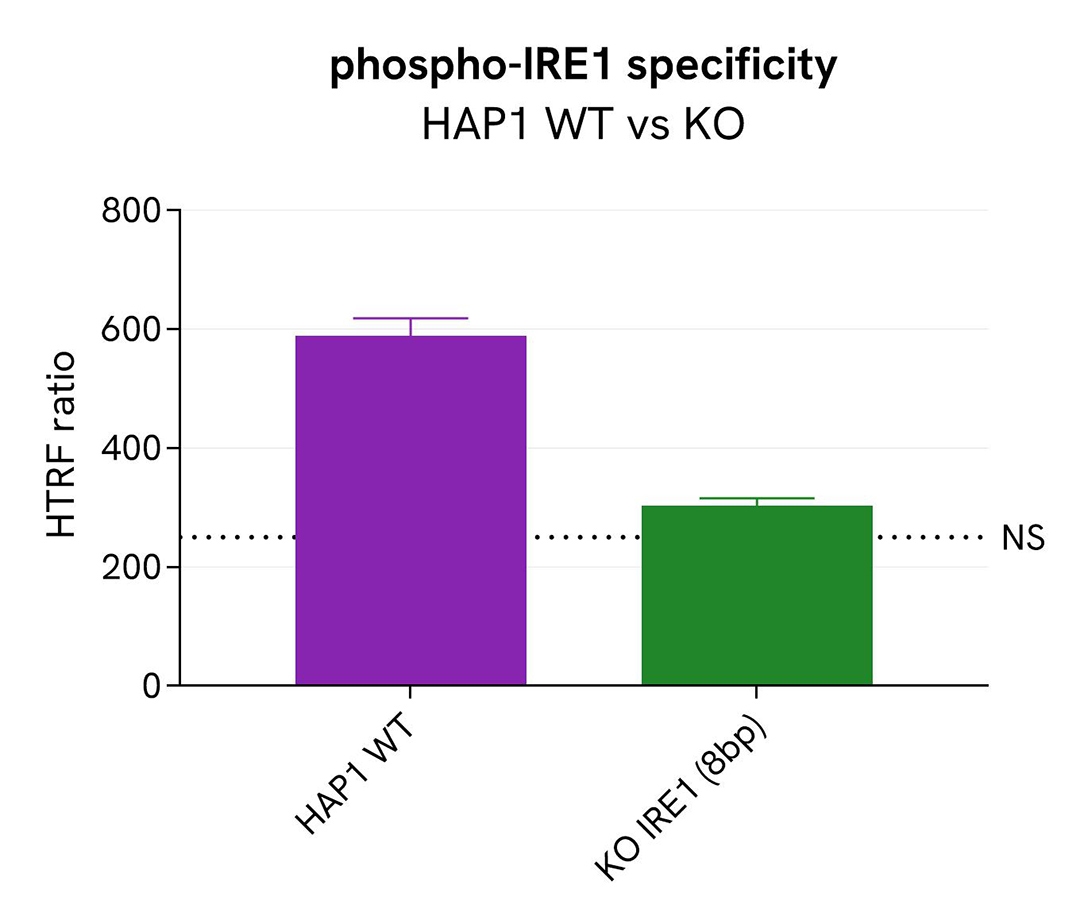
Validation of Phospho-IRE1 (Ser724) assay specificity
The Phospho-IRE1 (Ser724) protein levels were assessed with the HTRF Human Phospho-IRE1 (Ser724) kit in HAP1 cells (WT) and HAP1 cell line Knocked-Out for IRE1. Cell density was optimized beforehand to ensure HTRF detection within the dynamic range of the kit (data not shown).
The different cell lines were cultured in a 96-well plate (200,000 cells/well) for 24 hours at 37°C, 5% CO2. Following the 24h incubation, cells were treated with Thapsigargin (1µM, 45 mins). The cells were then lysed with 25 µL of supplemented lysis buffer #1 (1X) for 30 minutes at RT under gentle shaking, and 16 µL of cell lysate were transferred into a low volume white microplate followed by 4 µL of premixed detection reagents. The HTRF signal was recorded after 4 hours of incubation at RT.
In HAP1 KO IRE1 cells, the Phospho-IRE1 (Ser724) HTRF signal was equivalent to the non-specific signal (dotted line), demonstrating that the Phospho IRE1 assay is specific for IRE1.
Catalog cell line references (Horizon Discovery): HAP1 WT #C631; HAP1 KO IRE1 # HZGHC000742c006
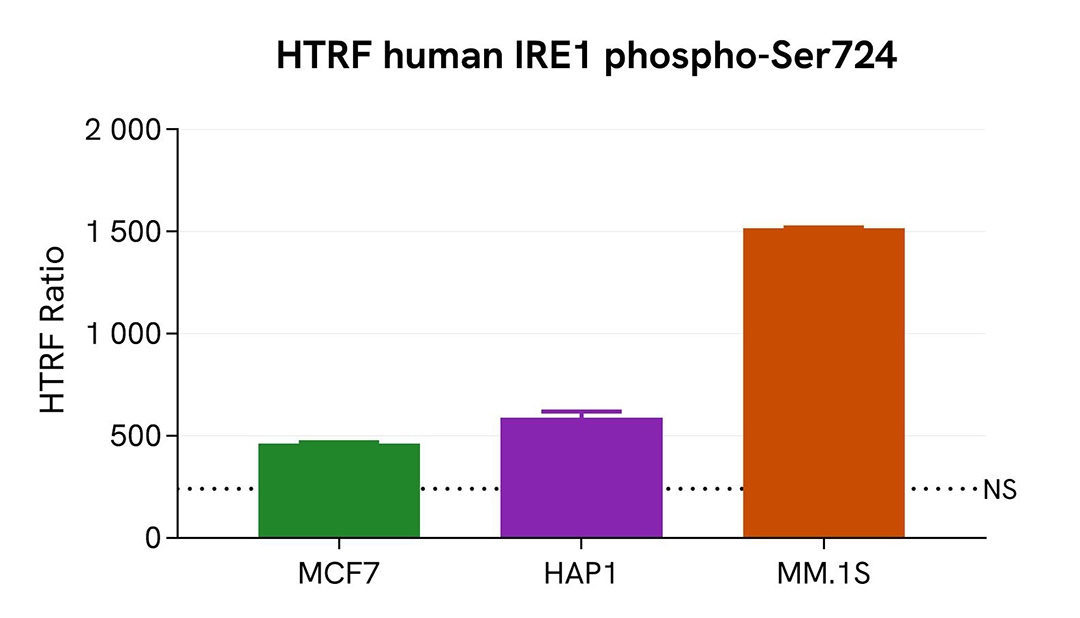
Assessment of Phospho-IRE1 (Ser724) level in various cell lines
Adherent (MCF7 & HAP1) and suspension (MM.1S) human cells were seeded at 200,000 cells/well in a 96-well microplate. After 24h of incubation, the cells were treated for 1 hour with 1 µM of Thapsigargin. They were next lysed for 30 minutes with supplemented lysis buffer #1, following the protocol for adherent or suspended cells at RT under gentle shaking.
16 µL of lysate were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF Phospho-IRE1 (S724) detection reagents. The HTRF signal was recorded after 4 hours of incubation.
The HTRF Phospho-IRE1 (Ser724) assay detected IRE1 phosphorylation at residue S724 in various cellular models expressing different levels of the protein.
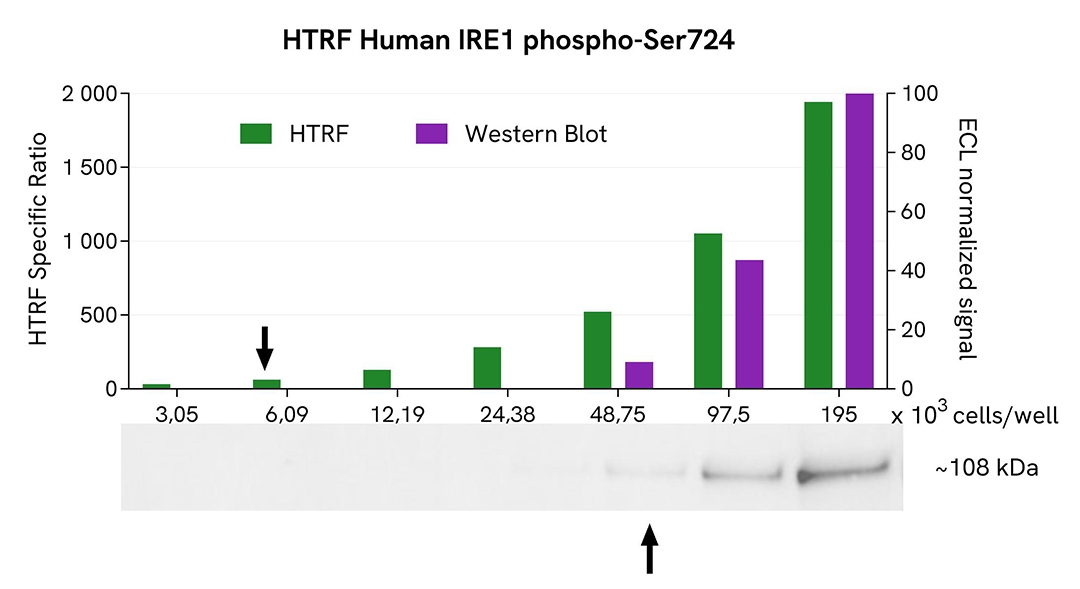
HTRF Phospho-IRE1 assay compared to Western Blot
MM.1S cells were cultured in a T175 flask and treated with 1µM Thapsigargin for 1 hour in medium at 37°C, 5% CO2. After medium removal, the cells were lysed with 3 mL of supplemented lysis buffer #1 (1X) for 30 min at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer #1 (1X), and 16µL of each dilution were transferred into a 384-well small volume microplate before the addition of 4µL of HTRF Phospho-IRE1 (S724) detection antibodies. HTRF signals were recorded after 4 hours of incubation.
Equal amounts of lysates were loaded into a gel for a side-by-side comparison between HTRF and Western Blot.
In these conditions, the HTRF Phospho IRE1 (S724) assay is 8-fold more sensitive than the Western Blot technique.
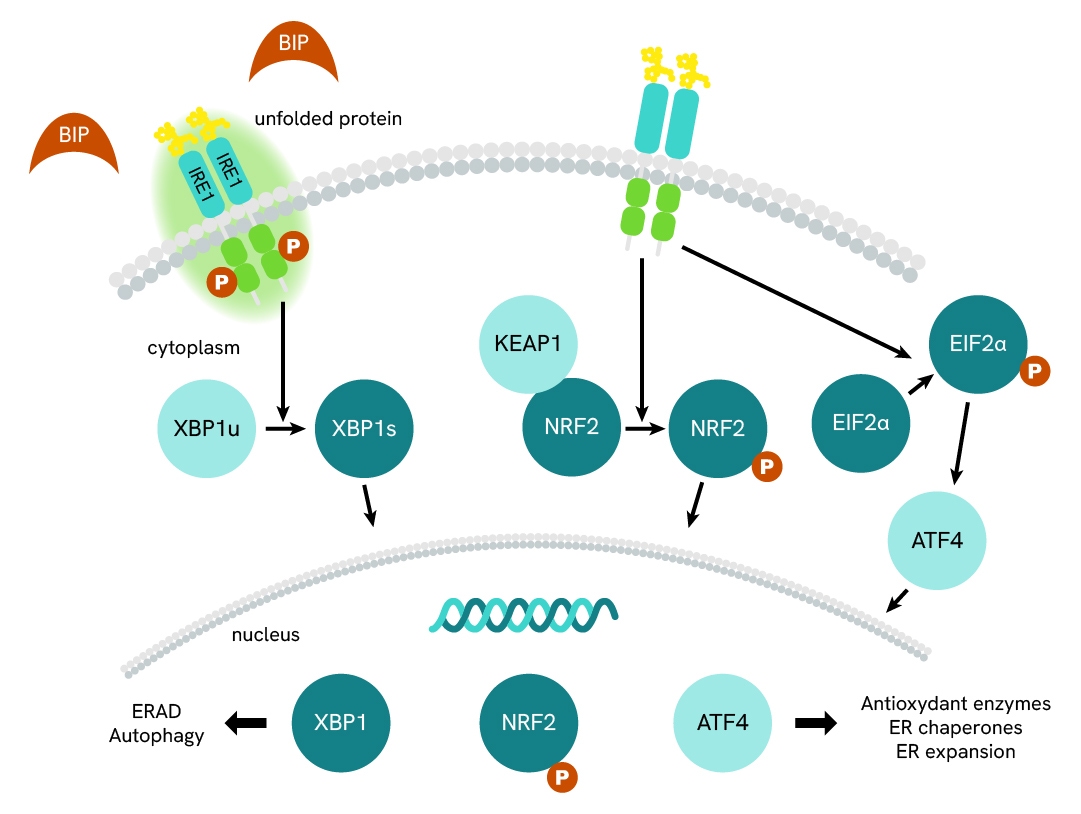
Simplified pathway
IRE1 signaling pathway
IRE1 is one of the main transducers of the Unfolded Protein Response signaling pathway with roles in regulation of ER stress and UPR (unfolded protein response). Upon ER stress and an accumulation of unfolded proteins, BiP releases its inhibitory ligand to activate IRE1. IRE1 receptor dimerizes then autophosphorylates, which activates its mRNase activity. Through this endoribonuclease activity, IRE1 degrades mRNAs (RIDD) and splices XBP-1u mRNA to XBP1s, enabling its translation and then its translocation to the nucleus. XBP-1s then acts as a transcription factor for chaperones to handle the overload of unfolded proteins.
Loading...


How can we help you?
We are here to answer your questions.






























