
HTRF Human Phospho-DDR1 (Tyr796) Detection Kit, 500 Assay Points

HTRF Human Phospho-DDR1 (Tyr796) Detection Kit, 500 Assay Points




This HTRF kit enables the cell-based quantitative detection of DDR1 when phosphorylated at Tyr796.
For research use only. Not for use in diagnostic procedures. All products to be used in accordance with applicable laws and regulations including without limitation, consumption and disposal requirements under European REACH regulations (EC 1907/2006).
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
This HTRF kit enables the cell-based quantitative detection of DDR1 when phosphorylated at Tyr796.
For research use only. Not for use in diagnostic procedures. All products to be used in accordance with applicable laws and regulations including without limitation, consumption and disposal requirements under European REACH regulations (EC 1907/2006).


HTRF Human Phospho-DDR1 (Tyr796) Detection Kit, 500 Assay Points


HTRF Human Phospho-DDR1 (Tyr796) Detection Kit, 500 Assay Points


Product information
Overview
DDR (Discoidin Domain Receptor family member 1) is a transmembrane tyrosine kinase receptor found in epithelial and mesenchymal cells. it binds and is activated by collagen and is a key player in cell-matrix interaction, with roles in adhesion and migration. These functions make it a potentail target of interest in matrix-related disorders such as fibrosis, but also some types of cancer wher cell migration and invasion is critical.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 4
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Technology |
TR-FRET
|
| Unit Size |
500 Assay Points
|
Assay validation
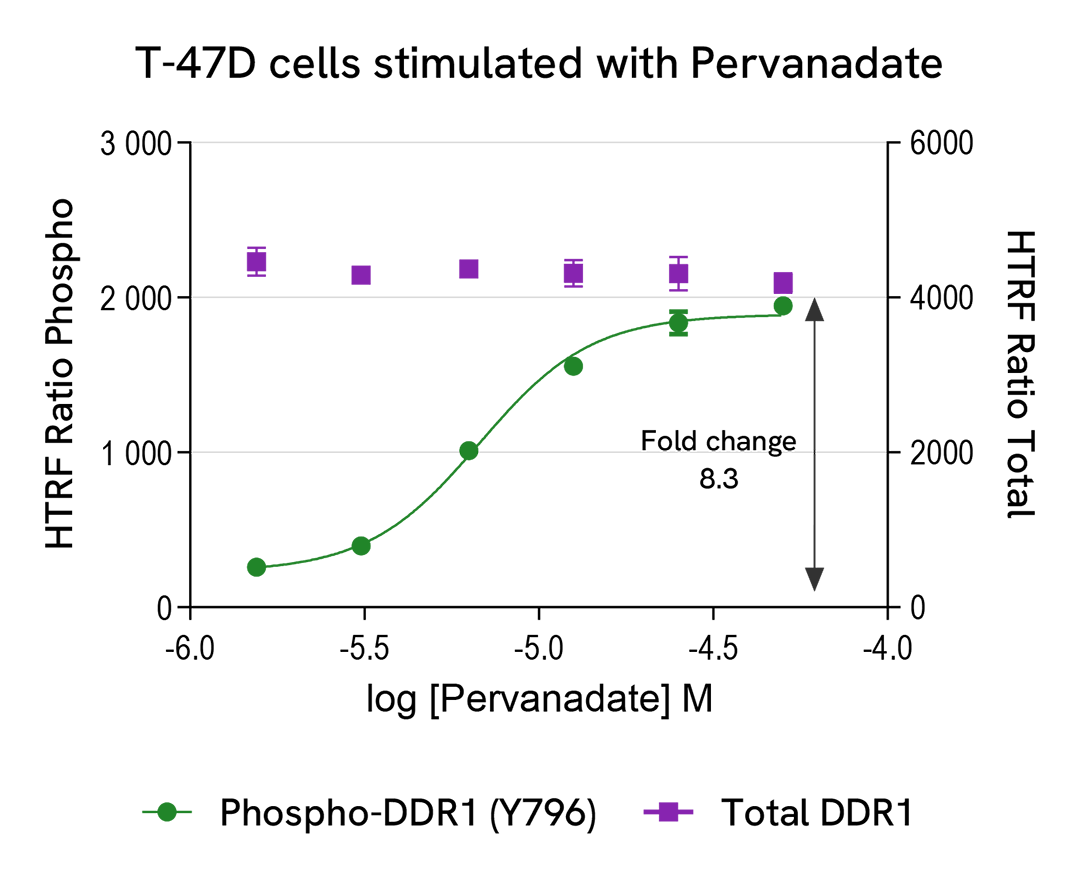
Induction of Phospho-DDR1 (Tyr796) in endogeneous and overexpressed DDR1 cellular models
T-47D cells were seeded in a 96-well culture-treated plate (100,000 cells/well) in complete culture medium, and incubated overnight at 37°C, 5% CO2. The cells were treated for 30 minutes with increasing concentrations of Pervanadate.
After treatment, the cells were lysed with 50 µL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking. For the detection step, 16 µL of cell lysate were transferred into a 384-well low volume white microplate and 4 µL of the HTRF Phospho-DDR1 (Tyr796) or Total DDR1 detection reagents were added. The HTRF signal was recorded after an overnight incubation.
As expected, Pervanadate triggered a dose-dependent increase in the level of Phospho-DDR1 (Tyr796).
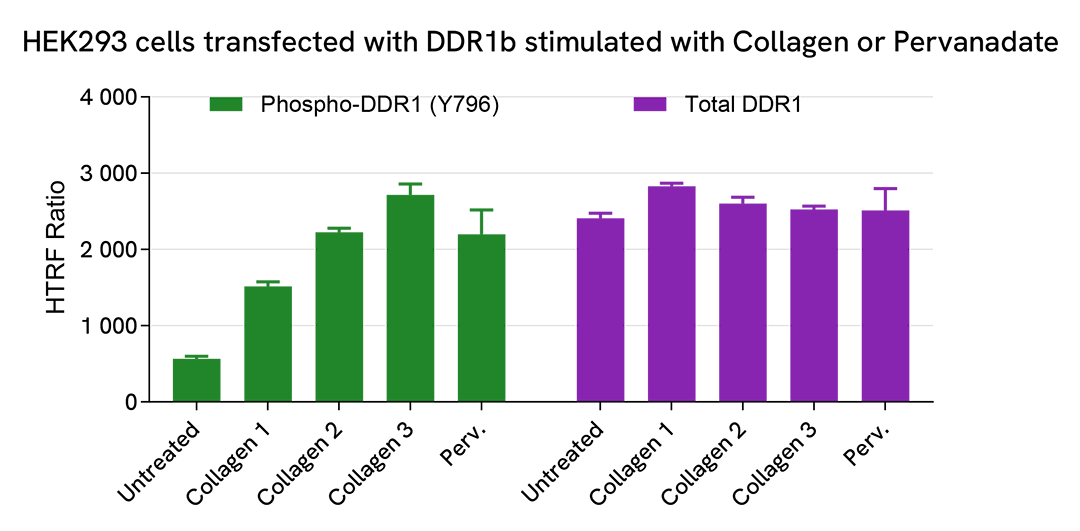
HEK293 cells were seeded in a 96-well culture-treated plate (100,000 cells/well) in complete culture medium, and incubated overnight at 37°C, 5% CO2. The cells were transfected with DDR1b using DharmaFECT kb (Horizon Discovery). After 24h of incubation, the cells were treated with increasing concentrations of Collagen (from C1 to C3: 0.625, 1.25, and 10 µg/mL) for 1h or with Pervanadate for 30 minutes (100 µM).
After treatment, the cells were lysed with 50 µL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking. For the detection step, cell lysates were diluted 1/20 for Total or 1/5 for Phospho (Tyr796), and then 16 µL were transferred into a 384-well low volume white microplate. 4 µL of the HTRF Phospho-DDR1 (Tyr796) or Total DDR1 detection reagents were added. The HTRF signal was recorded after an overnight incubation.
As expected, Collagen (Endogeneous ligand of DDR1 receptor) triggered a dose-dependent increase in the level of Phospho-DDR1 (Tyr796), as did Pervanadate.

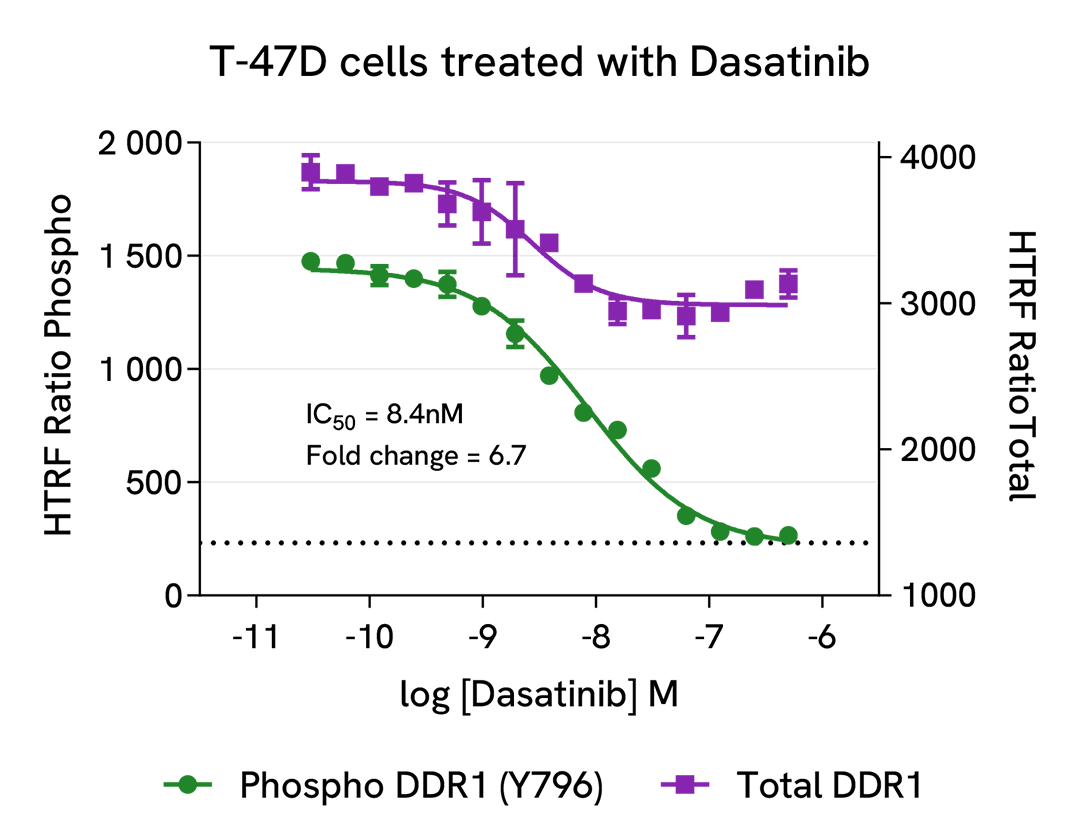
Inhibition of Phospho-DDR1 (Tyr796) in endogeneous and overexpressed DDR1 cellular models
T-47D cells were seeded in a 96-well culture-treated plate (100,000 cells/well) in complete culture medium, and incubated overnight at 37°C, 5% CO2. The cells were treated for 4 hours with increasing concentrations of Dasatinib, and 100 µM Pervanadate were added 30 minutes before the end of the treatment. The cells were lysed with 50 µL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking.
For the detection step, 16 µL of cell lysate were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF Phospho-DDR1 (Tyr796) or Total-DDR1 detection reagents were added. The HTRF signal was recorded after an overnight incubation.
As expected, the DDR1 kinase inhibitor Dasatinib induced a dose-dependent decrease in DDR1 phosphorylation, but also a slight inhibition in the expression level of the receptor, while no toxicity was detected (ATPlite Luminescence Assay System, #6016943).
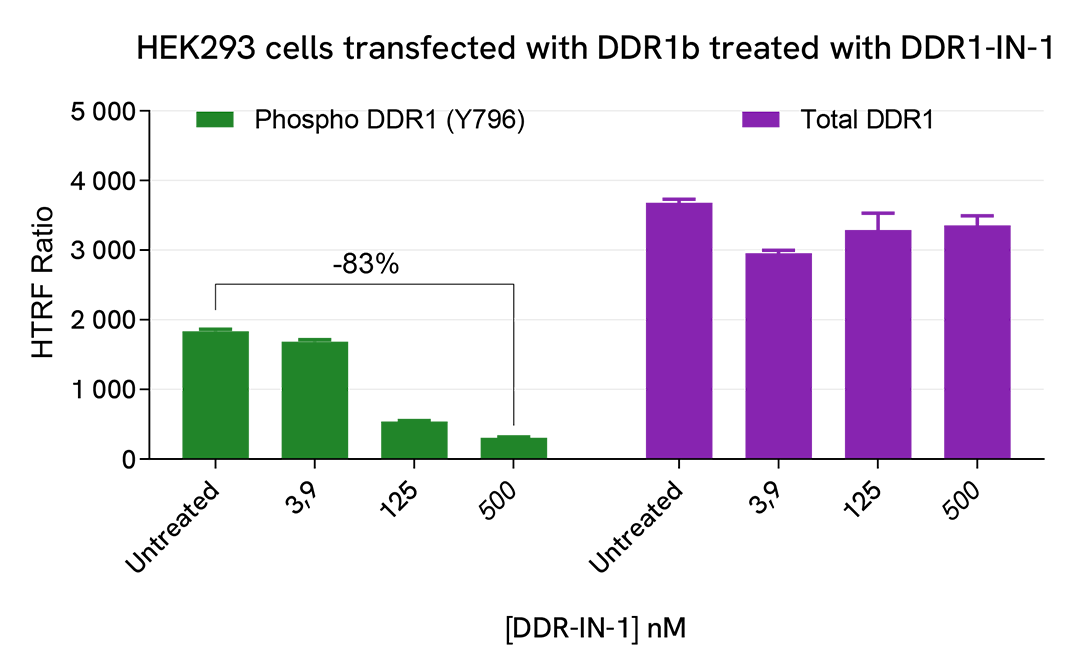
HEK293 cells were seeded in a 96-well culture-treated plate (100,000 cells/well) in complete culture medium, and incubated overnight at 37 °C, 5% CO2. The cells were transfected with DDR1b using DharmaFECT kb (Horizon Discovery). After 24h of incubation, the cells were treated for 2 hours with increasing doses of DDR1-IN-1, and 10 µg/mL Collagen were added 1h before the end of the treatment.
The cells were lysed with 50 µL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking. For the detection step, cell lysates were 1/20 for Total or 1/5 for Phospho (Tyr796) and then 16 µL were transferred into a 384-well low volume white microplate. 4 µL of the HTRF Phospho-DDR1 (Tyr796) or Total-DDR1 detection reagents were added. The HTRF signal was recorded after an overnight incubation.
As expected, the DDR1 kinase inhibitor DDR1-IN-1 induced a dose-dependent decrease in DDR1 phosphorylation, without effect on the expression level of the receptor.
Selectivity of Phospho-DDR1 (Tyr796) assay using transfection of different isoforms of DDR1 & DDR2
HEK293 cells were plated in a 96-well plate (25,000 cells/well) and cultured for 24h. The cells were then transfected with different plasmids, DDR1a, DDR1b, or DDR2, as well as with a negative control. Following a 24h incubation, cells were treated with Pervanadate (100 µM, 30 min).
After cell lysis, 16 µL of lysates were transferred into a 384-well low volume white microplate and 4 µL of the HTRF Phospho-DDR1 (Tyr796) detection antibodies were added. The HTRF signal was recorded after an overnight incubation.
Cell transfection with DDR1a and DDR1b led to the detection of Phospho-DDR1 (Tyr796) compared to the negative control. On the contrary, the transfection of DDR2 did not induce any signal increase, demonstrating that the Phospho-DDR1 (Tyr796) assay is specific for DDR1a and DDR1b, and does not cross-react with a DDR2 family member.
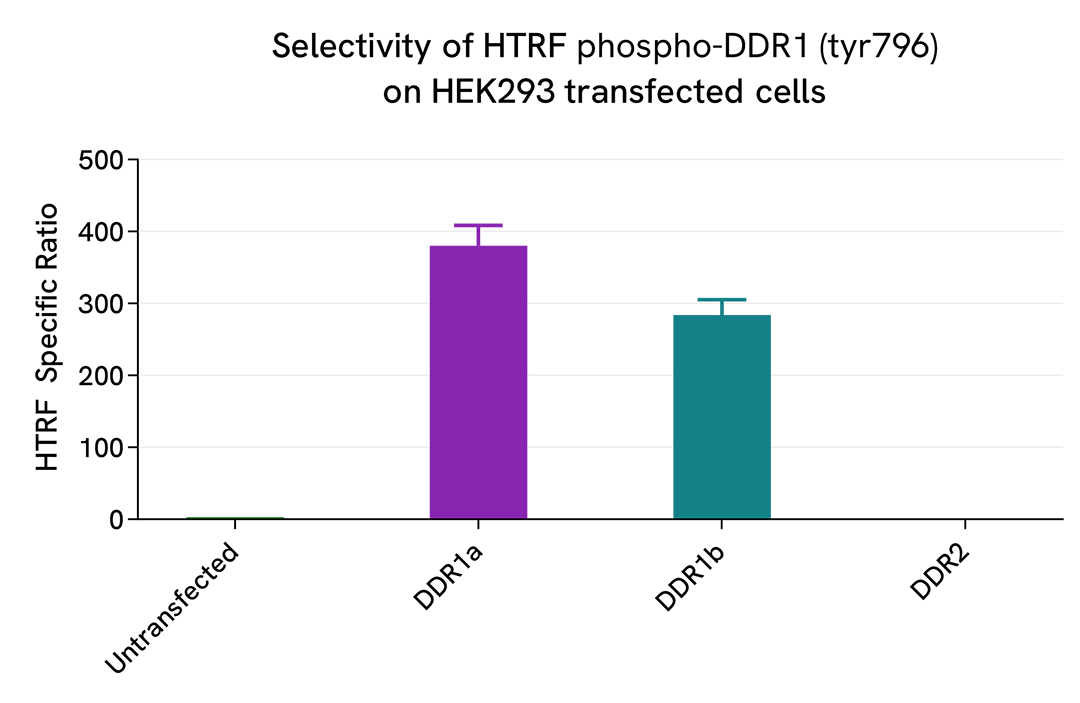
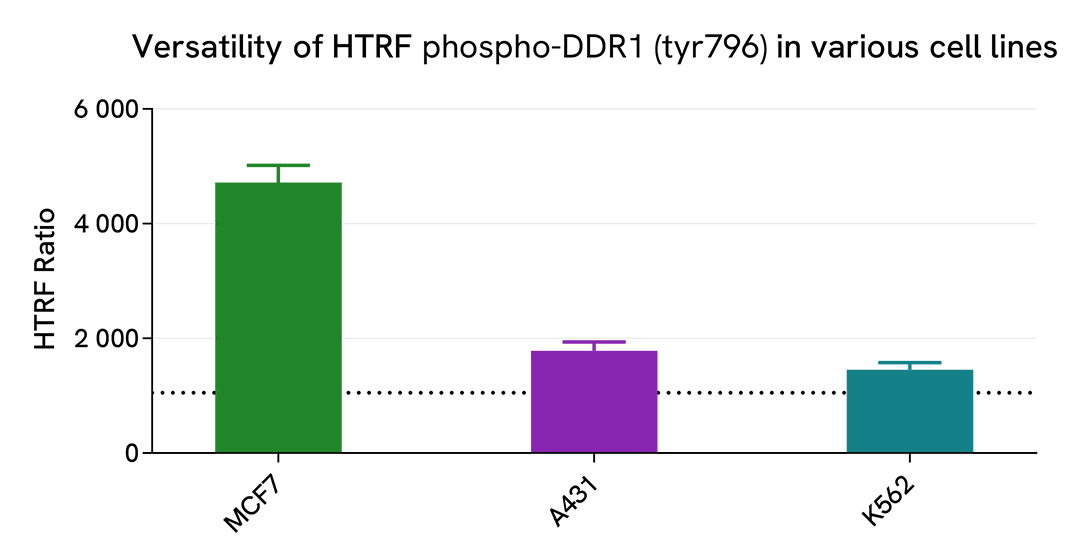
Assessment of Phospho-DDR1 (Tyr796) levels in various human cell lines
Adherent (MCF7 & A431) and suspension (K562) human cells were seeded at respectively 100,000 and 200,000 cells/well in a 96-well microplate. After 24h of incubation, the cells were treated for 30 min with 100 µM of Pervanadate before being lysed for 30 minutes with supplemented lysis buffer #4, following the protocol for adherent or suspended cells, at RT under gentle shaking.
16 µL of lysate were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF Phospho-DDR1 (Tyr796) detection reagents. The HTRF signal was recorded after an overnight incubation.
The HTRF Phospho-DDR1 (Tyr796) assay detected DDR1 phosphorylation at residue Y796 in various cellular models, with different phosphorylation levels.
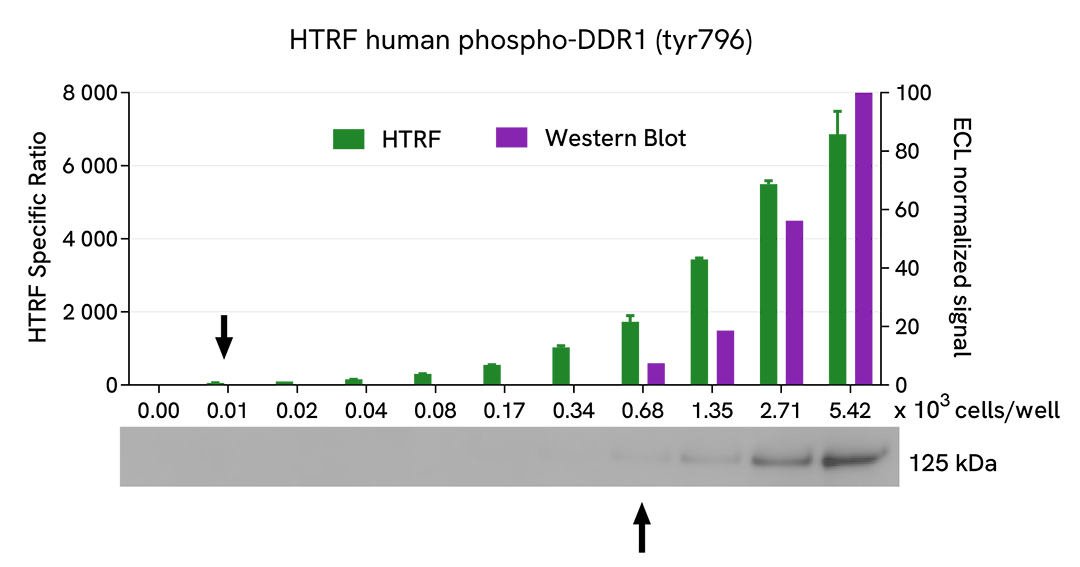
HTRF DDR1 Phospho-Y796 assay compared to Western Blot
HEK293 cells were grown in a T175 flask in complete culture medium at 37°C - 5% CO2 until 80% confluence. Transfection of DDR1b was performed with DharmaFECT kb (Horizon Discovery). After 24h of incubation, the cells were treated for 30 min with 100 µM of Pervanadate before being lysed with 3 mL of supplemented lysis buffer #4 (1X) for 30 minutes at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer, and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF Phospho-DDR1 (Tyr796) detection reagents. Equal amounts of lysates were used for a side-by-side comparison between HTRF and Western Blot.
Using the HTRF Phospho-DDR1 (Tyr796) assay, 10 cells/well were enough to detect a significant signal, while 680 cells were needed to obtain a minimal chemiluminescent signal using Western Blot. Therefore, in these conditions, the HTRF Phospho-DDR1 (Tyr796) assay was 64 times more sensitive than the Western Blot technique.
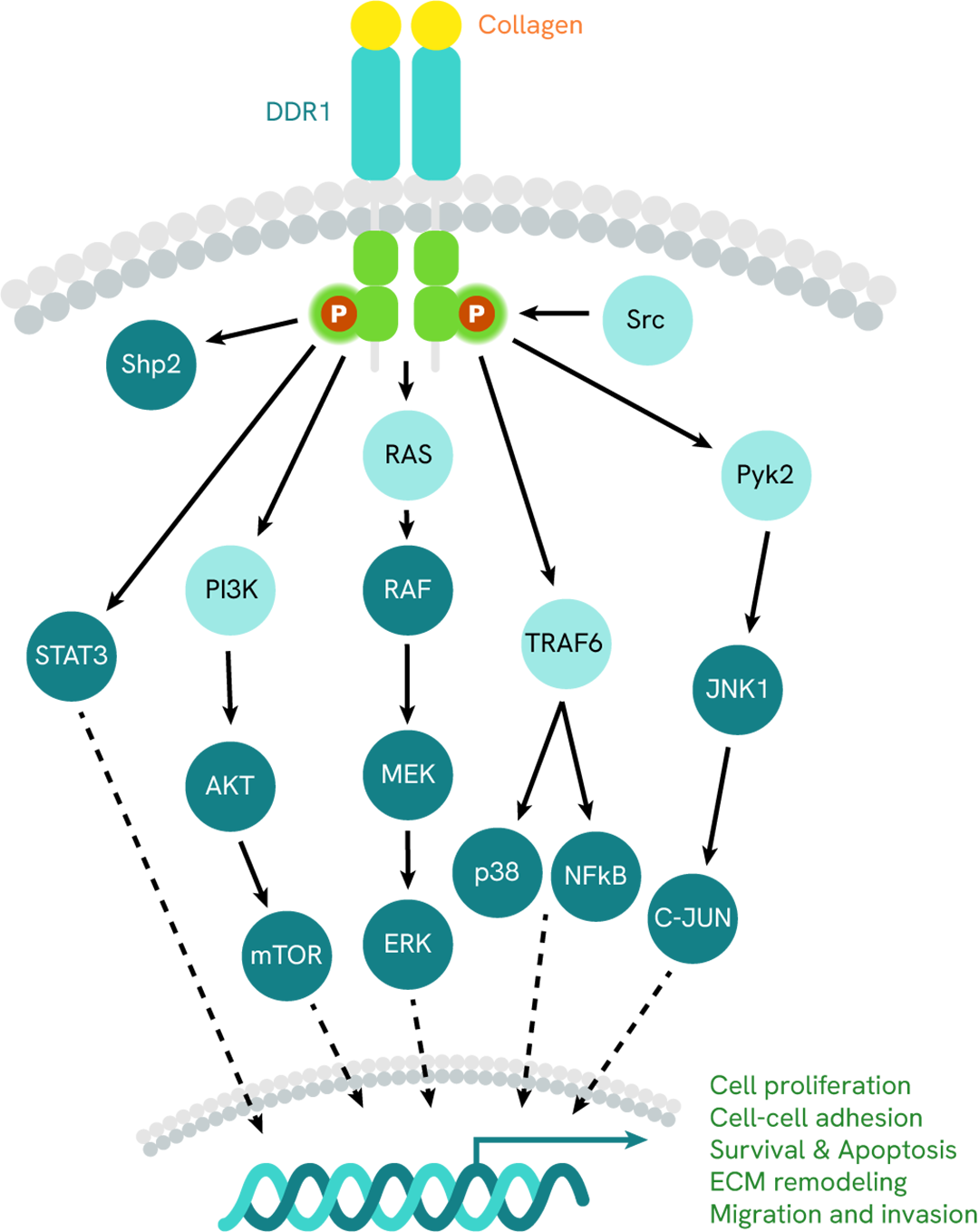
Simplified pathway
DDR1 signaling pathway
DDR1 is activated by collagen binding that triggers its dimerization into a complex that in turn autophosphorylates on multiple intracellular tyrosines. Phosphorylated tyrosine residues serve as docking sites for various adaptor proteins. These induce the activation of downstream signaling pathways (MAPK, PI3K/AKT, STAT3, Pyk2, or TRAF6...) necessary for cell proliferation, adhesion, migration, and ECM remodeling.
Resources
Are you looking for resources, click on the resource type to explore further.
HTRF: unfamiliar territory?
This technical brochure reviews the general principles of HTRF™ and the associated Tag-lite™ technology...
Your guide for improving cell signaling assay performance
This complete Revvity guide provides you with all the tools you need to...


How can we help you?
We are here to answer your questions.






























