
HTRF Human Total DAP12 Detection Kit, 500 assay points
HTRF Human Total DAP12 Detection Kit, 500 assay points


This HTRF kit allows for the cell-based quantitative detection of total DAP12.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
This HTRF kit allows for the cell-based quantitative detection of total DAP12.


HTRF Human Total DAP12 Detection Kit, 500 assay points


HTRF Human Total DAP12 Detection Kit, 500 assay points


Product information
Overview
The Total DAP12 cellular assay monitors total DAP12 protein levels and can be used as a normalization assay in conjunction with our Phospho-DAP12 (Tyr91) kit. This kit is designed to be compatible with the buffers from the Phospho-DAP12 kit, allowing the same lysate to be used for analyses of both the phosphorylated and the total protein populations. DAP12 (DNAX-Activation Protein 12), also known as TYROBP (TYRO protein tyrosine kinase-binding protein), is a transmembrane signaling adaptor protein that plays a crucial role in innate immune responses and neuroinflammation. DAP12 is widely expressed in various immune cells, including natural killer cells, monocytes, macrophages, and microglia, the resident immune cells of the central nervous system.
The DAP12-TREM2 signaling axis is particularly important in the context of neuroinflammation and neurodegenerative diseases. It regulates microglial activation and function, influencing the brain's immune response and homeostasis. Genetic variants of TREM2 have been identified as risk factors for Alzheimer's Disease, highlighting the significance of this pathway in maintaining brain health. Monitoring total DAP12 levels alongside its phosphorylated form provides valuable insights into the overall expression and activation state of this crucial signaling protein.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 3
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target |
DAP12
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Central Nervous System
|
| Unit Size |
500 assay points
|
How it works
Total DAP12 assay principle
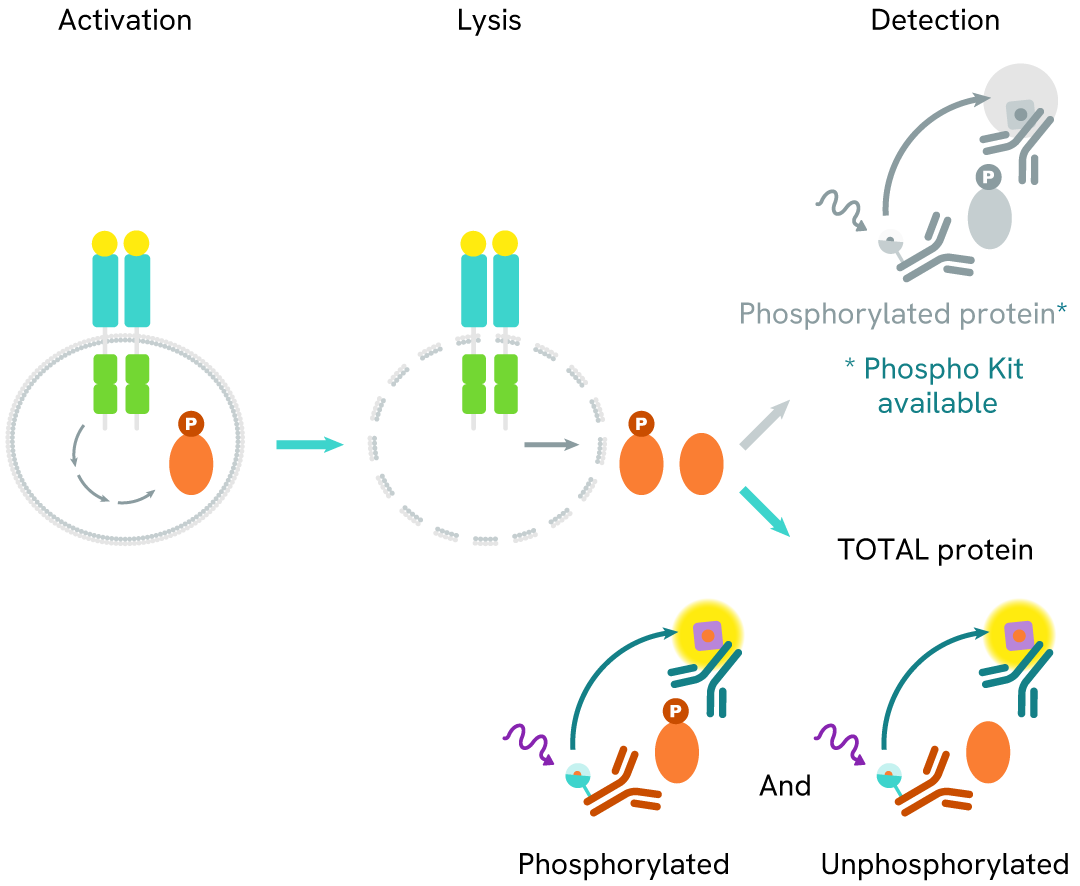
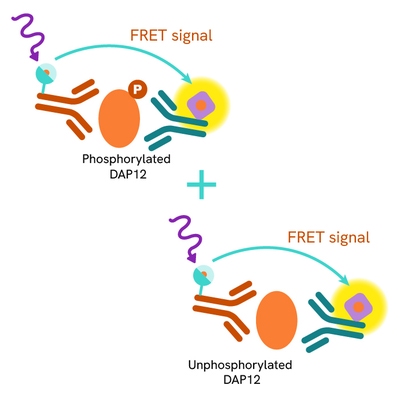
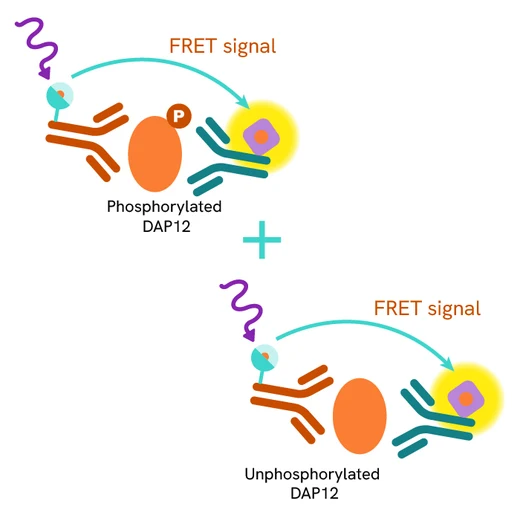
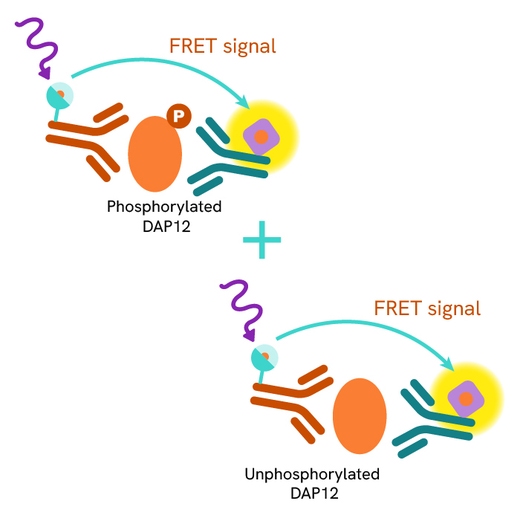
The Total DAP12 assay quantifies the expression level of DAP12 in a cell lysate. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Total DAP12 assay uses two labeled antibodies, one coupled to a donor fluorophore and the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of DAP12 this enables an immune-complex formation involving both labeled antibodies, and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal.
Its intensity is directly proportional to the concentration of total protein present in the sample and provides a means of assessing the protein's phosphorylation state under a no-wash assay format.

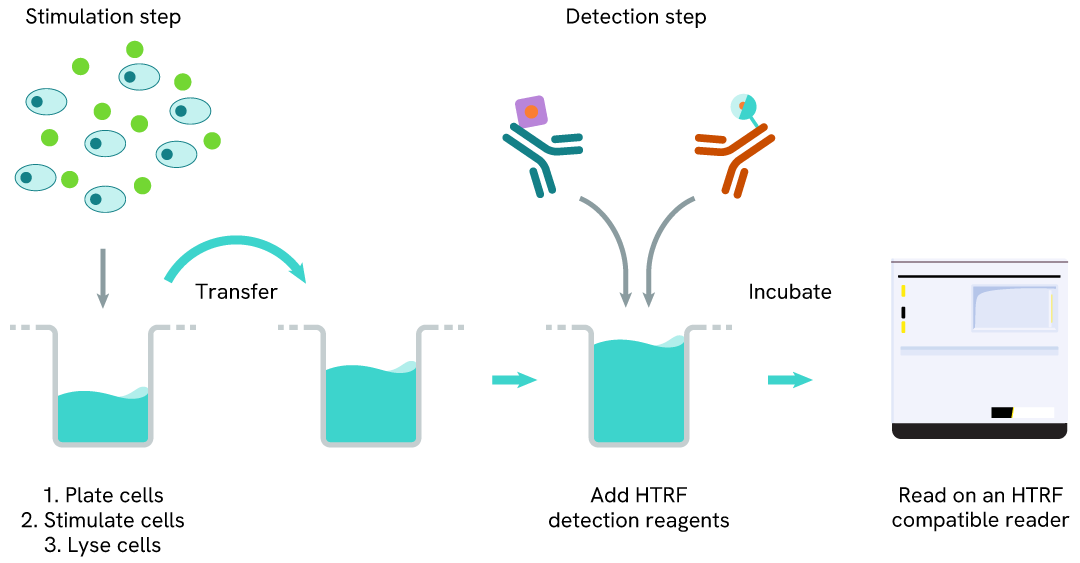
Total DAP12 two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of HTRF Total DAP12 detection reagents. This protocol allows for the cells' viability and confluence to be monitored.
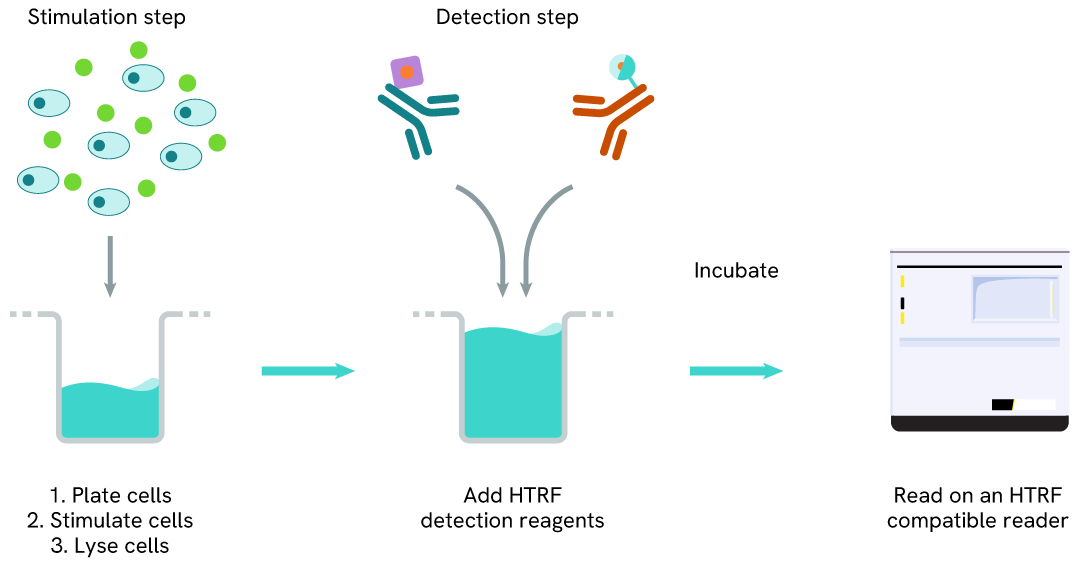
Total DAP12 one-plate assay protocol
Detection of Total DAP12 with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol allows miniaturization while maintaining robust HTRF quality.
Assay validation
Induction of Phospho-DAP12 (Tyr91) in an endogenous cellular model
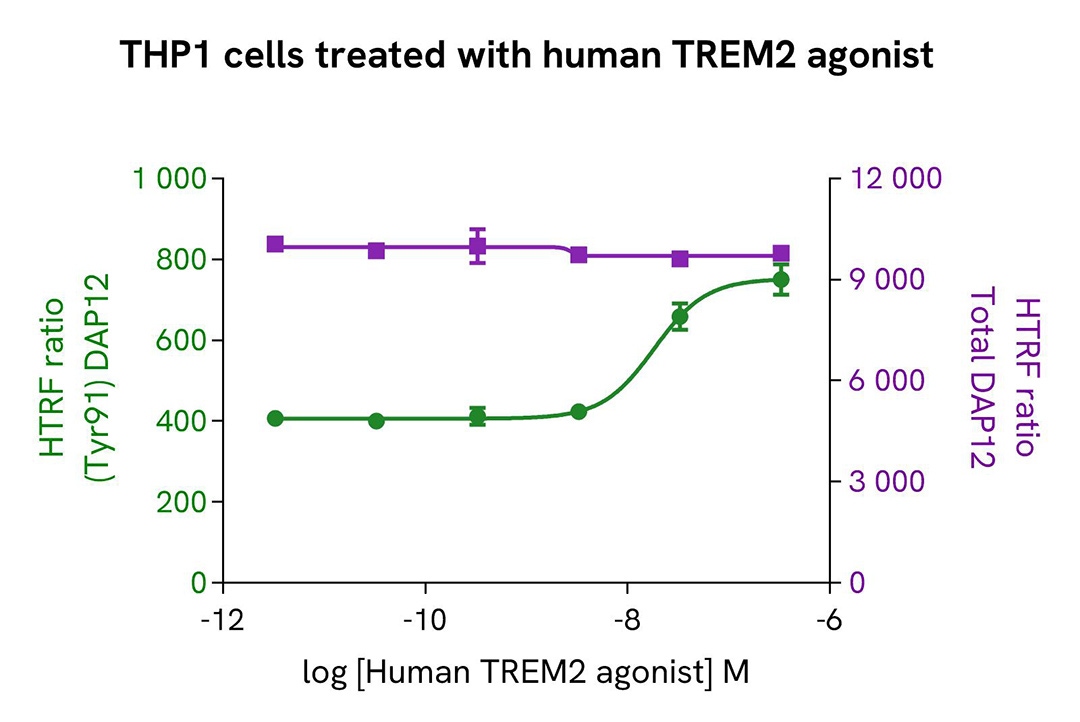
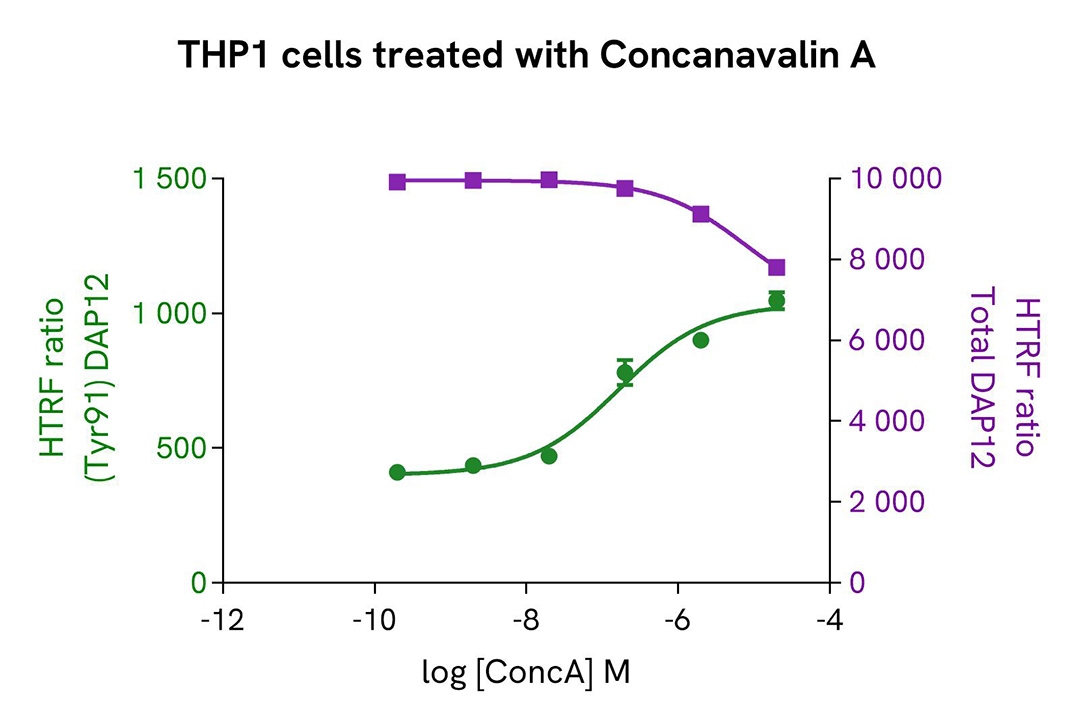
THP-1 cells were harvested and seeded at a density of 0.5 x 106 cells in 35 mL of RPMI complete medium in a T175 flask, and then treated with 35 ng/mL of TGFβ for 18 hours at 37°C and 5% CO2. After being scraped off, the THP-1 cells were centrifuged at 300 RCF for 3 minutes.
The cells were next seeded in a 96-well culture-treated plate (100,000 cells/well) in complete culture medium. They were pre-incubated with increasing concentrations of human TREM2 agonist or Concanavalin A for 10 minutes, before stimulation with 250 µM of Pervanadate for 10 minutes.
After treatment, the cells were lysed with 10 µL of supplemented lysis buffer #3 (4x) for 30 minutes at room temperature under gentle shaking. For the detection step, 16 µL of cell lysate were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF Phospho-DAP12 (Tyr91) or Total DAP12 detection reagents were added. The HTRF signal was recorded after an overnight incubation.
As expected, the two compounds induced activation of the TREM2 pathway and triggered a dose-dependent increase in the level of Phospho-DAP12 (Tyr91) without significant modulation of Total DAP12.
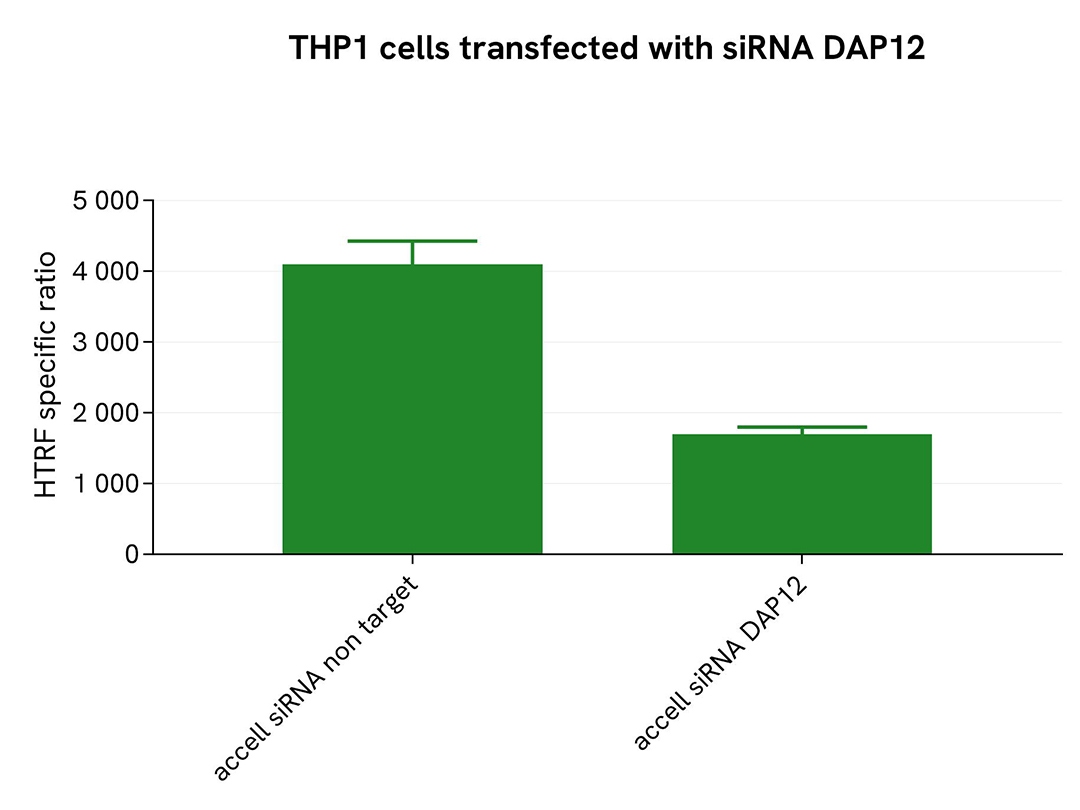
Validation of the selectivity of Total-DAP12 assay using Accell siRNA
THP-1 cells were plated in a 96-well plate (50,000 cells/well) and transfected with Accell siRNA DAP12, as well as a negative control. Following a 96-hour incubation, 250 µM of Pervanadate were added to the cells for 30 minutes before lysing with supplemented lysis buffer #3 (4x) for 30 minutes at room temperature under gentle shaking.
For the detection step, 16 µL of lysates were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF Total-DAP12 detection antibodies were added. The HTRF signal was recorded after 24 hours.
Cell transfection with siRNA DAP12 led to a significant decrease (59%) in the detection of the protein compared to the negative control (non-targeting control), demonstrating that the HTRF Total-DAP12 assay is selective.
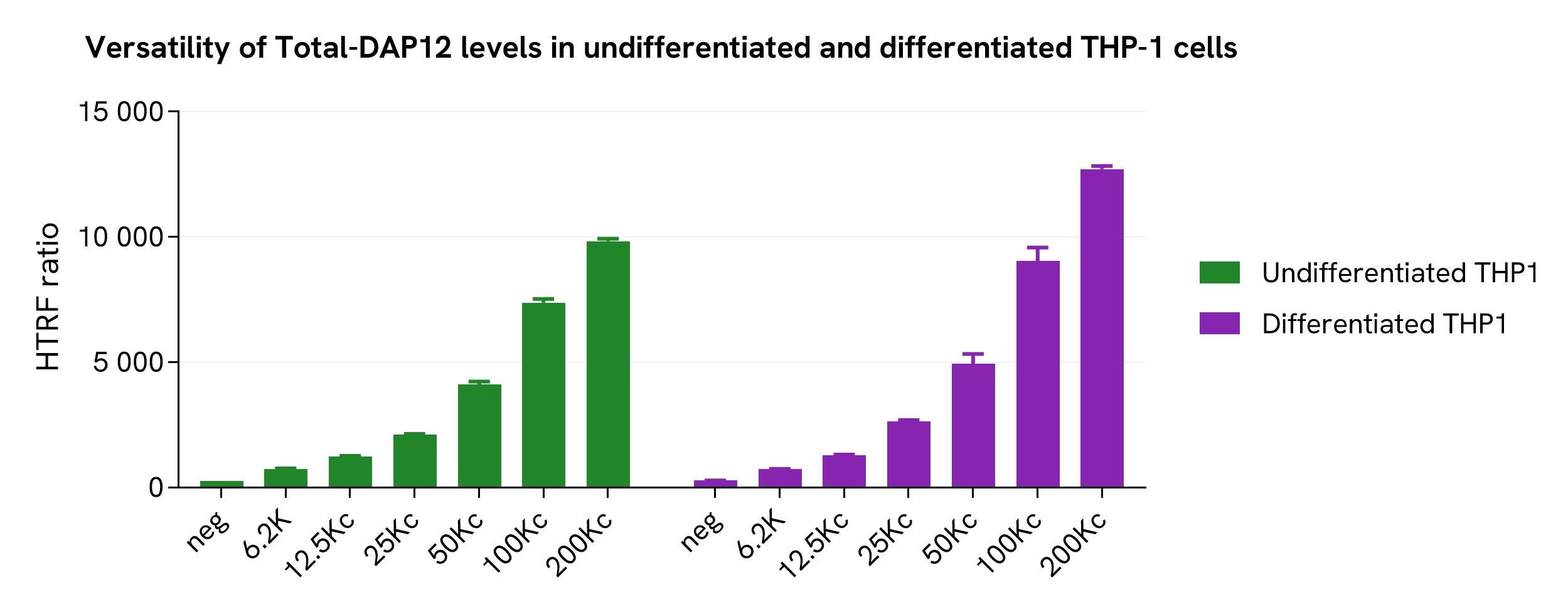
Assessment of Total-DAP12 level in undifferentiated and differentiated THP-1 cells
Various cell densities of undifferentiated and differentiated THP-1 cells (treated with 150 nM PMA for 20 hours) were seeded in a 96-well microplate. The cells were then treated with 250 µM of Pervanadate for 30 minutes before being lysed with supplemented lysis buffer #3 for 30 minutes at room temperature under gentle shaking.
Next, 16 µL of the lysate were transferred into a 384-well low volume white microplate, followed by the addition of 4 µL of the HTRF Total-DAP12 detection reagents. The HTRF signal was recorded after an overnight incubation.
The HTRF Total-DAP12 assay detected relatively the same amount of DAP12 in both monocytes and macrophage-like cells.
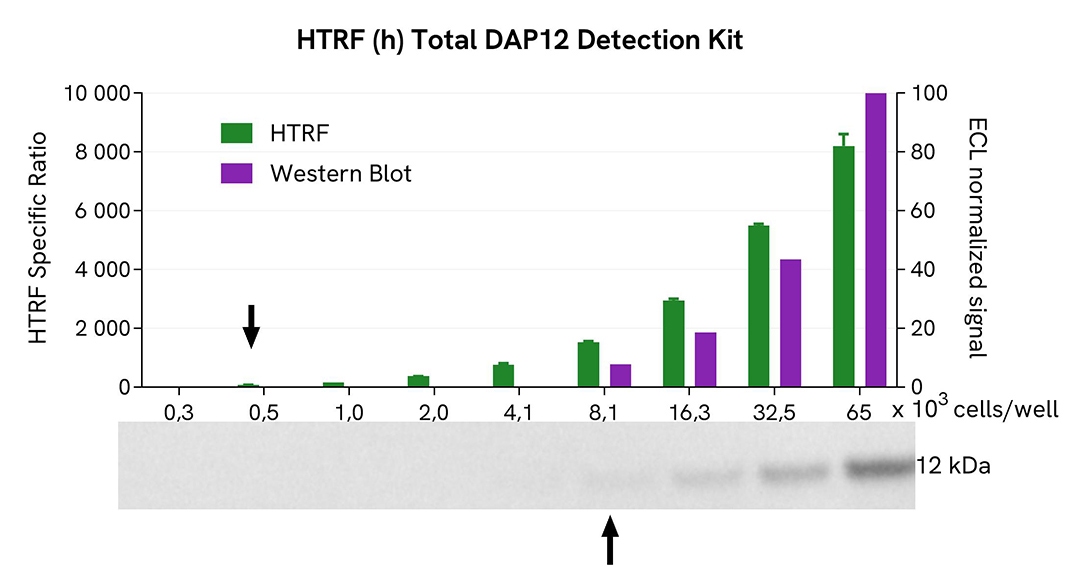
HTRF Total-DAP12 assay compared to Western Blot
THP-1 cells were grown in a T175 flask in complete culture medium at 37°C and 5% CO2 until they reached 80% confluence. The cells were then treated with 250 µM of Pervanadate for 30 minutes and lysed with 3 mL of supplemented lysis buffer #3 (4x) for 30 minutes at room temperature under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer. Then 13 µL of each dilution were transferred into a low volume white microplate, followed by the addition of 3 µL of supplemented lysis buffer #3 (1x) to ensure the same quantity for both techniques. Finally, 4 µL of HTRF Total-DAP12 detection reagents were added. Equal amounts of lysates were used for a side-by-side comparison between HTRF and Western Blot.
Using the HTRF Total-DAP12 assay, 500 cells/well were sufficient to detect a significant signal, while 8100 cells were needed to obtain a minimal chemiluminescent signal using Western Blot. Therefore, under these conditions, the HTRF Total-DAP12 assay was 16 times more sensitive than the Western Blot technique.
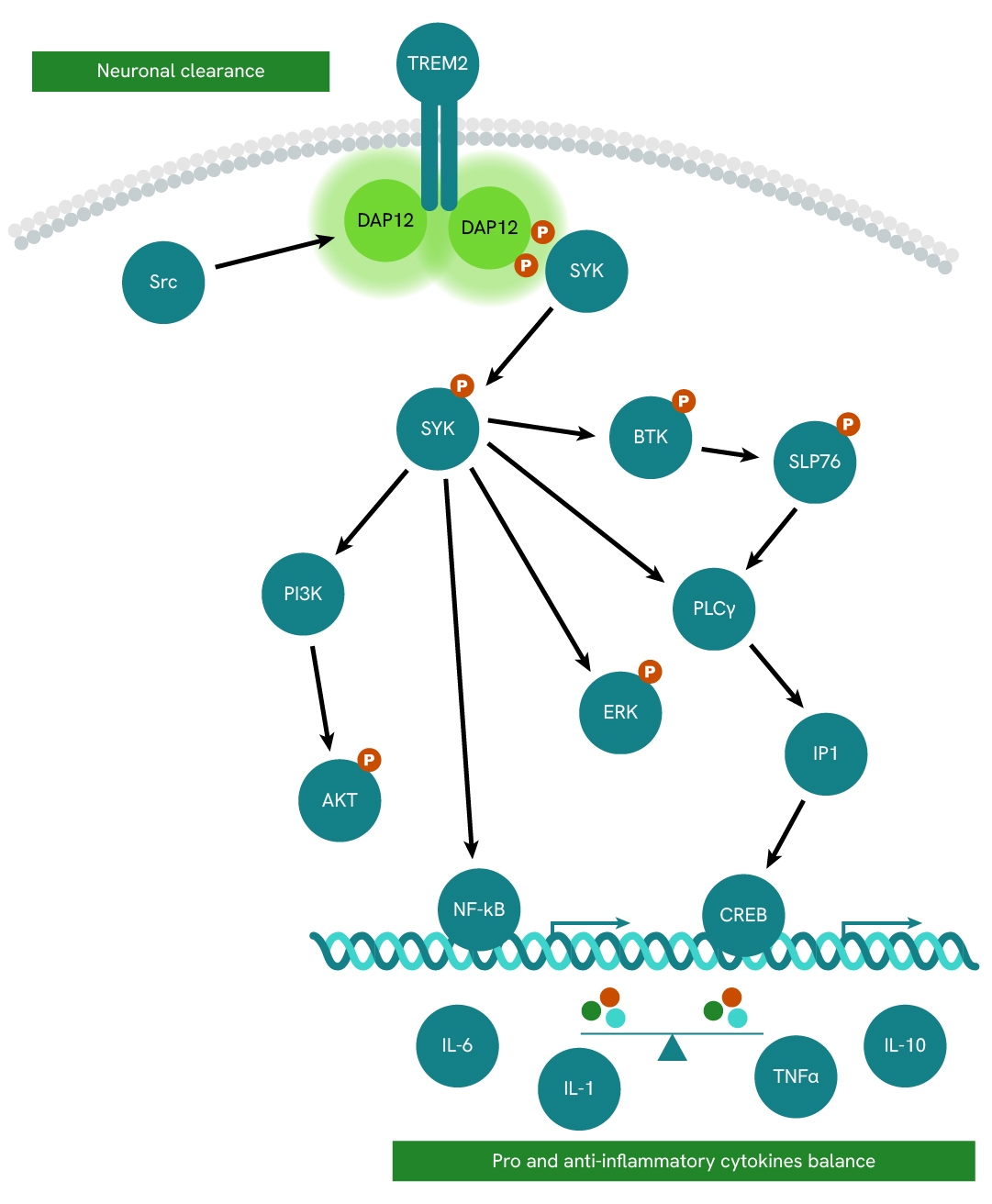
Simplified pathway
TREM2/DAP12 signaling pathway
The TREM2-DAP12 receptor complex plays a continuous surveillance role by scanning the neuronal microenvironment and maintaining healthy brain homeostasis in microglia cells.
DAP12 (also known as TYROBP or KARAP) is a homodimer transmembrane adaptor protein initially described as a receptor-activating subunit component of natural killer (NK) cells. It is expressed in various cell types, including monocytes, macrophages, dendritic cells, and microglia.
Due to its short extracellular domain, DAP12 itself is thought to lack ligand-binding capability. Instead, it forms complexes with ligand-binding receptors (DAP12-associated receptors) and transduces signals from these receptors into the cytoplasm.
Activation of TREM2 leads to DAP12 phosphorylation and subsequent recruitment of SYK, which promotes the phosphorylation and activation of multiple downstream mediators such as PI3K, AKT, BTK, ERK, and NFkβ.
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
Loading...


How can we help you?
We are here to answer your questions.






























