
HTRF Human Phospho-Cyclin B1 (Ser126) Detection Kit, 500 Assay Points

| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |



Product information
Overview
This HTRF cell-based assay provides a convenient and accurate way to detect phosphorylated Cyclin B1 at Ser126.
Cyclin B family members (including Cyclin B1 and Cyclin B2) are important regulators of cell cycle progression. They are integral mediators of the growth factor-dependent transition from G2 to Mitosis phases, playing the role of the regulatory subunit of CDK1.
Cyclin B1 is upregulated in the presence of extracellular mitogenic stimuli and is subsequently rapidly downregulated by proteasomal degradation following its phosphorylation on Ser126.
Cyclin B1 is often overexpressed in many cancers. Such abnormal overexpression of Cyclin B1 results in dysregulated CDK1 activity and can lead to tumorigenesis.
How it works
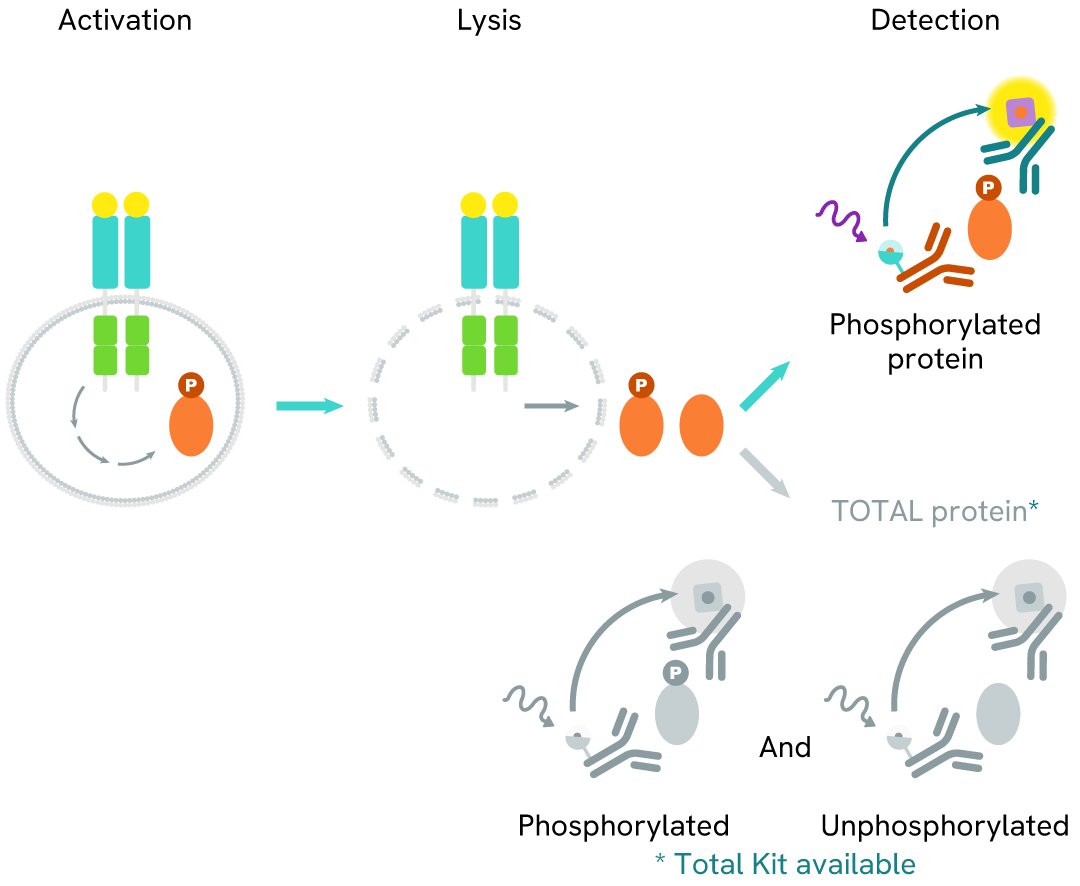
Phospho-Cyclin B1 (Ser126) assay principle
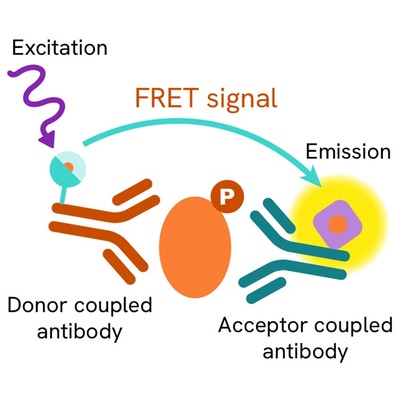
The Phospho-Cyclin B1 (Ser126) assay measures Cyclin B1 when phosphorylated at Ser126. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The assay uses 2 antibodies, one labeled with a donor fluorophore and the other with an acceptor. The first antibody was selected for its specific binding to the phosphorylated motif on the protein, and the second for its ability to recognize the protein independently of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies. This brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the protein's phosphorylation state under a no-wash assay format.

Phospho-Cyclin B1 (Ser126) two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of Phospho-Cyclin B1 (Ser126) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.
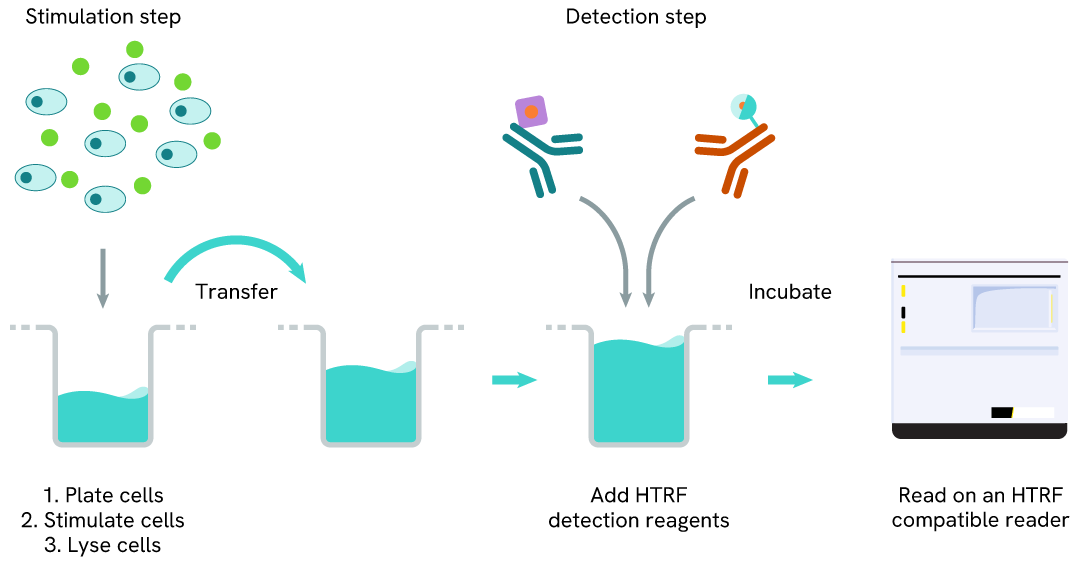
Phospho-Cyclin B1 (Ser126) one-plate assay protocol
Detection of Phosphorylated Cyclin B1 (Ser126) with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.
Assay validation
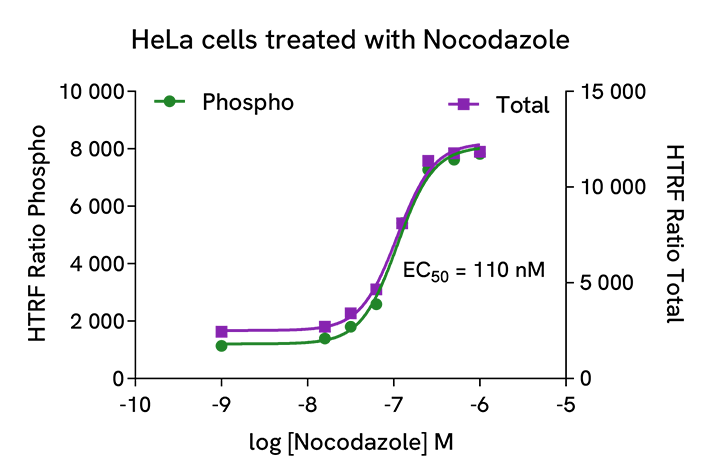
Phospho-Cyclin B1 (Ser126) modulation using Nocodazole
HeLa cells were cultured in a 96-well plate (20,000 cells/well) for 24h and then treated overnight with increasing concentrations of Nocodazole (cell cycle blocker halting cells at the G2/M border). After cell lysis, 16 µL of lysates were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF Phospho-Cyclin B1 (Ser126) or Total Cyclin B1 detection antibodies were added. The HTRF signal was recorded after an overnight incubation.
As expected, Nocodazole triggered a dose-dependent increase in total and phosphorylated Cyclin B1 at Ser126.
Pharmacological Modulation of Phospho-Cyclin B1 (Ser126)
HeLa cells were cultured in a 96-well plate (20,000 cells/well), treated overnight with 100 nM of Nocodazole (halting cells at the G2/M border), then treated or not with 10 µM of MG132 for 30 min, followed or not by the addition of 9 µM of RO-3306 for 2 h.
After cell lysis, 16 µL of lysates were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF Phospho-Cyclin B1 (Ser126) or Total Cyclin B1 detection antibodies were added. The HTRF signal was recorded after an overnight incubation.
As expected, Nocodazole with MG132 treatment blocks the cell cycle in G2/M, inducing an accumulation of phospho-Cyclin B1(Ser126) and Total Cyclin B1.
A co-treatment of Nocodazole and CDK1 inhibitor (RO-3306) induces a decrease in phospho (Ser126) and Total Cyclin B1 at a basal level, consistent with Cyclin B1 degradation.
In the latter condition, proteasome blocking with MG132 restores the level of phospho (Ser126) and Total Cyclin B1 by inhibiting Cyclin B1 degradation, as described by Vassilev et al., 2006. In fact, by inhibiting CDK1-Cyclin B1 activity RO-3306 could promote an exit from mitosis leading to a degradation of Cyclin B1.
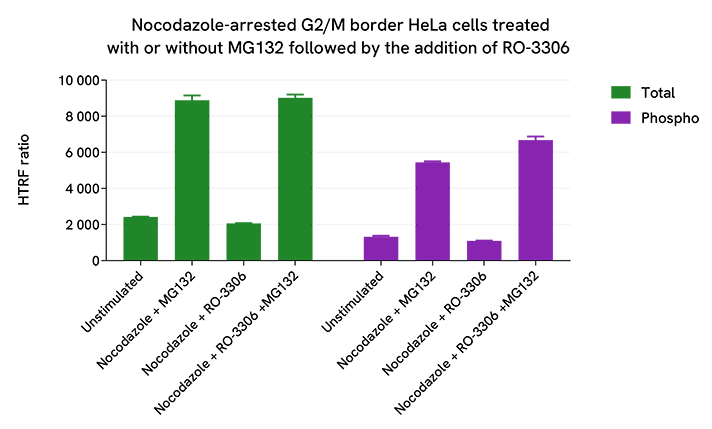
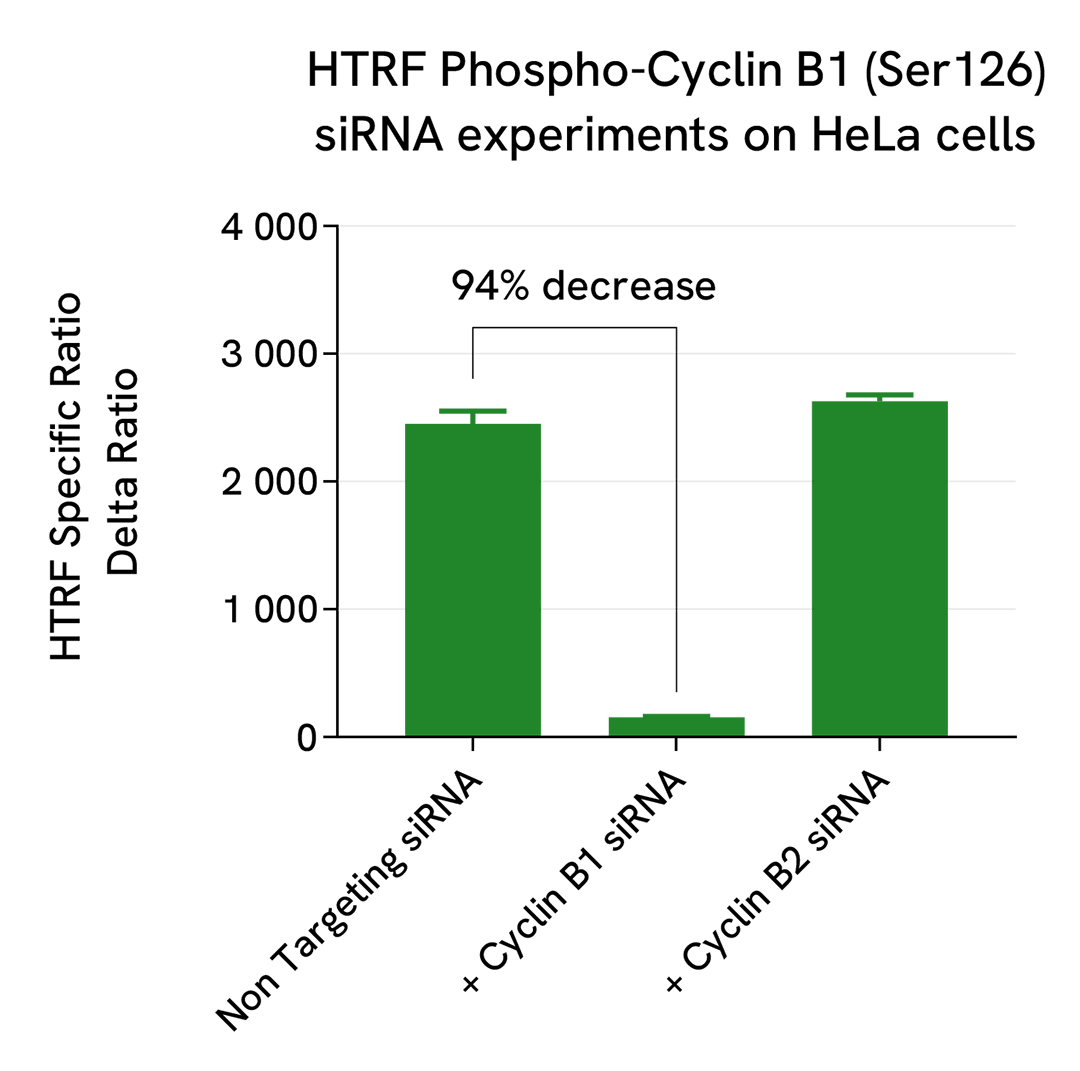
Specificity of Phospho Cyclin B1 (Ser126) assay using siRNA knockdown experiments
HeLa cells were plated in a 96-well plate (20,000 cells/well) and cultured for 24h. The cells were then transfected with siRNAs specific for Cyclin B1 or Cyclin B2 as well as with a negative control siRNA. Following a 24h incubation, the medium was removed, and the cells were lysed. 16 µL of lysates were transferred into a 384-well low volume white microplate and 4 µL of the HTRF Phospho-Cyclin B1 (Ser126) detection antibodies were added. The HTRF signal was recorded after an overnight incubation.
Cell transfection with the Cyclin B1 siRNA led to a 94% signal decrease compared to the cells transfected with the negative siRNA. On the contrary, the knockdown of Cyclin B2 did not induce any signal decrease, demonstrating that the HTRF Phospho-Cyclin B1 (Ser126) assay is specific for Cyclin B1 phosphorylation and does not cross-react with other Cyclin B family members.
Assessment of Phospho-Cyclin B1 (Ser126) levels in various cell lines
The adherent human cells HeLa, MCF7, and SH-SY5Y were seeded at 50,000 cells/well in a 96-well microplate.
After a 24h incubation, the cells were lysed with supplemented lysis buffer. 16 µL of lysate were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF Phospho Cyclin B1 (Ser126) detection reagents. The HTRF signal was recorded after an overnight incubation.
The HTRF Phospho Cyclin B1 (Ser126) assay efficiently detected Phospho Cyclin B1 (Ser126) in various cellular models expressing different levels of the protein.

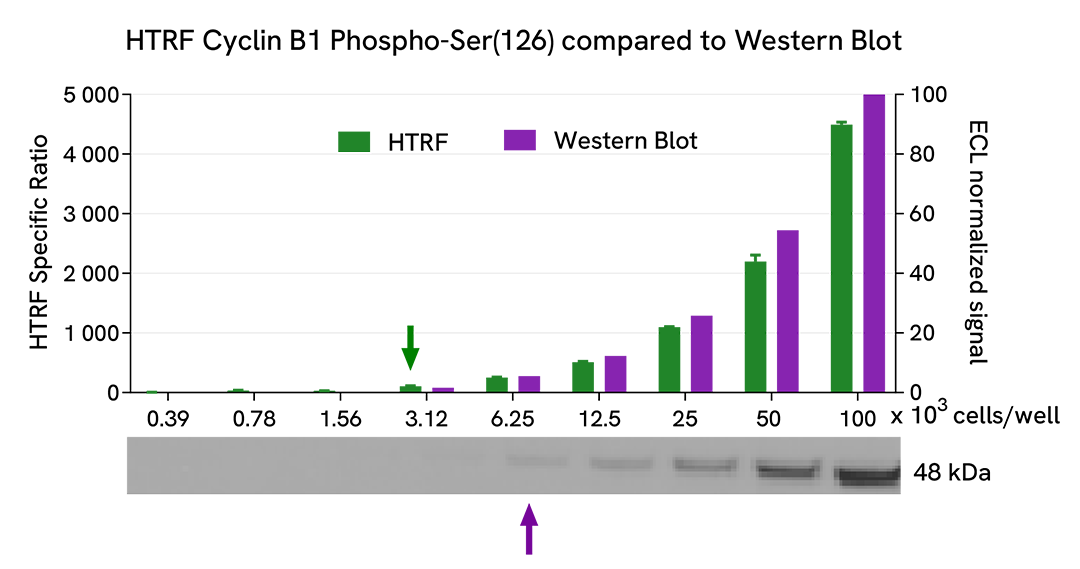
HTRF Cyclin B1 phospho-Ser126 assay compared to Western Blot
HeLa cells were cultured in a T175 flask in complete culture medium at 37°C, 5% CO2. After 48h of incubation, the cells were lysed with 3 mL of supplemented lysis buffer #1 (1X) for 30 minutes at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer, and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF Phospho Cyclin B1 (Ser126) detection reagents. Equal amounts of lysates were used for a side-by-side comparison between HTRF and Western Blot.
Using the HTRF Phospho Cyclin B1 (Ser126) assay, 3120 cells/well were enough to detect a significant signal, while 6250 cells were needed to obtain a minimal chemiluminescent signal using Western Blot. Therefore, in these conditions, the HTRF Phospho Cyclin B1 (Ser126) assay was twice as sensitive as the Western Blot technique.
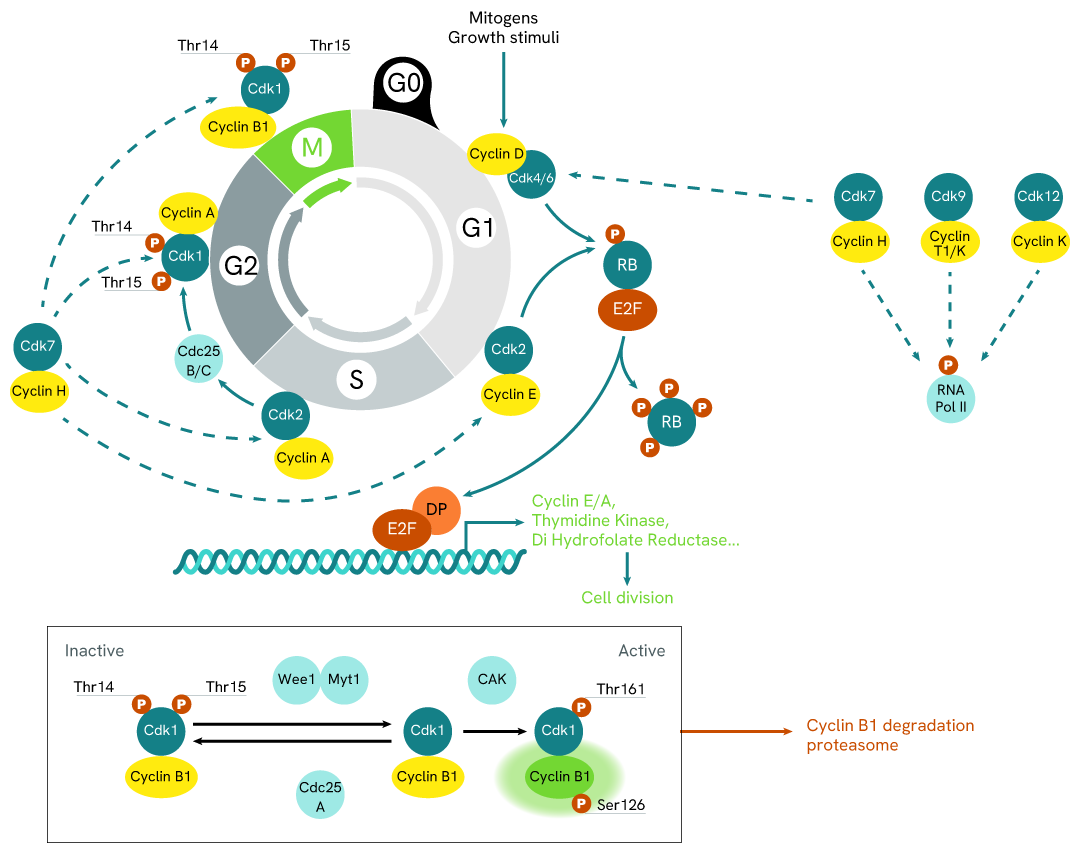
Simplified pathway
Cyclin B1 assay Signaling Pathway
Cyclin B1 is a protein involved in the regulation of cell cycle progression through the G2/M-phase checkpoint allowing mitosis.
Synthesis of cyclin B1 during the S and G2 phases and the activation of CDK1 via Thr161 phosphorylation by CAK leads to the assembly of Cdk1–Cyclin B1 heterodimers that translocates to the nucleus. The entry of cells into mitosis is preceded by activation of CDK1, and phosphorylation of Cyclin B1 at the Ser126 position.
Once in the nucleus, this activated complex then phosphorylates mitotic substrate proteins, and triggers the initiation of mitotic changes like chromosome condensation and nuclear membrane breakdown, essential for cells to enter mitosis.
The degradation of cyclin B1 is required for inactivation of the kinase and exit from mitosis. In this way Cyclin B1 is degraded by the proteasome through the the ubiquitin pathway.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Technology |
TR-FRET
|
| Unit Size |
500 assay points
|
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...


Loading...
How can we help you?
We are here to answer your questions.






























