

HTRF Human Phospho-Aurora A (Thr288) Detection Kit, 500 Assay Points

HTRF Human Phospho-Aurora A (Thr288) Detection Kit, 500 Assay Points




This HTRF kit enables the cell-based quantitative detection of phosphorylated human Aurora A at Thr288.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
This HTRF kit enables the cell-based quantitative detection of phosphorylated human Aurora A at Thr288.



HTRF Human Phospho-Aurora A (Thr288) Detection Kit, 500 Assay Points



HTRF Human Phospho-Aurora A (Thr288) Detection Kit, 500 Assay Points



Product information
Overview
This HTRF cell-based assay conveniently and accurately detects human Aurora A protein (related to the AURKA gene) ubiquitously expressed in eukaryotes. Aurora A belongs to the serine/threonine Aurora kinase family (including Aurora B and Aurora C), essential for cell division via mitosis regulation especially in the process of chromosomal segregation. It is activated through the autophosphorylation of Threonine 288 residue mainly occurring from the late S-Phase through the M phase during the cellular division cycle or DNA damage response. The overexpression of the Aurora kinases in a wide range of cancers (Leukemia, Colon Cancer, Prostate Cancer, and Breast Cancer) makes them interesting candidates for the development of Aurora kinase inhibitors (AKIs).
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 4
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Unit Size |
500 assay points
|
Video gallery

HTRF Human Phospho-Aurora A (Thr288) Detection Kit, 500 Assay Points

HTRF Human Phospho-Aurora A (Thr288) Detection Kit, 500 Assay Points

How it works
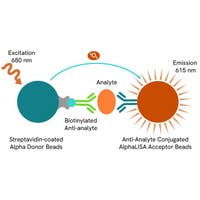
Phospho-Aurora A(Thr288) assay principle
The Phospho-Aurora A (Thr288) assay measures Aurora A when phosphorylated at Thr288. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Phospho-Aurora A (Thr288) assay uses 2 labeled antibodies: one with a donor fluorophore, the other with an acceptor. The first antibody was selected for its specific binding to the phosphorylated motif on the protein, the second for its ability to recognize the protein independent of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the protein’s phosphorylation state under a no-wash assay format.

Phospho-Aurora A (Thr288) 2-plate assay protocol
The 2-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before adding Phospho-Aurora A (Thr288) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Phospho-Aurora A(Thr288) 1-plate assay protocol
Detection of Phosphorylated Aurora A (Thr288) with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
Activation of total and Phospho-Aurora A (Thr288) measured on HeLa cells
HeLa cells (Immortalized Human cervical cancer cell line) were seeded in a 96-well culture-treated plate at 100,000 cells/well for 24 hours in complete culture medium for adhesion. After cell culture medium removal, they were treated with increasing concentrations of Nocodazole for 20h at 37° C, 5% CO2. After overnight treatment (20h), the cell culture medium was removed and 50 µl of supplemented Lysis Buffer#1 (1X) were dispensed into each well for 30 min at RT under gentle shaking. After cell lysis, 16 µL of lysates were transferred into a 384-well low volume white microplate and 4 µL of the HTRF Total Aurora A or Phospho-Aurora A (Thr288) detection antibodies were added. The HTRF signal was recorded after an overnight incubation. Nocodazole is a microtubule destabilizer inducing a G2/M cell cycle arrest. It has been demonstrated that Nocodazole-treated cells show higher Aurora protein expression levels, to overcome cell cycle arrest [1]. As expected, the results obtained show an increased level of Total and Thr288 Phosphorylated Aurora A proteins upon treatment with Nocodazole.
[1] : Jiang et al. Oncogene,2003

Inhibitor characterization with Phospho-Aurora A (Thr288) and total aurora a on HeLa cells


HeLa cells (Immortalized Human cervical cancer cell line) were seeded in a 96-well culture-treated plate at 100,000 cells/well for 24 hours in complete culture medium for adhesion. After cell culture medium removal, they were treated with increasing concentrations of inhibitors ( MLN8054, Tozasertib, or Alisertib) co-incubated with a fixed concentration of Nocodazole at 200 nM for 20h at 37° C, 5% CO2, in cell culture medium containing 5% SVF. After overnight treatment, the cell culture medium was removed and 50 µl of supplemented Lysis Buffer#1 (1X) were dispensed into each well for 30 min at RT under gentle shaking. After cell lysis, 16 µL of lysates were transferred into a 384-well low volume white microplate and 4 µL of the HTRF Total Aurora A or Phospho-Aurora A (Thr288) detection antibodies were added. The HTRF signal was recorded after an overnight incubation.
The 3 compounds have been described in the literature for their inhibitory effect on phosphorylated Aurora A (thr288), reaching a 98% maximum inhibition, without impacting the total protein level [2,3,4,5].
As expected, the results obtained show a clear dose-dependent inhibition of the Aurora A phosphorylation at Thr288 upon treatment with MLN8054, Tozasertib, or Alisertib, while the Aurora A protein expression level remains constant.
[2] : Manfredi et al. PNAS,2006
[3] : Sells et al. ACS Med.Chem.Lett.,2015
[4] : Tyler et al. Cell cycle,2007
[5] : Qi et al. Leuk Res,2013

Phospho-Aurora A Inhibitors Versus Phospho-Aurora B profiling


HeLa cells (Immortalized Human cervical cancer cell line) were seeded in a 96-well culture-treated plate at 100,000 cells/well for 24 hours in complete culture medium for adhesion. After cell culture medium removal, they were treated with increasing concentrations of inhibitors ( MLN8054, Tozasertib, or Alisertib) co-incubated with a fixed concentration of Nocodazole at 200 nM for 20h at 37 ° C, 5% CO2, in cell culture medium containing 5% SVF. After overnight treatment, the cell culture medium was removed and 50 µl of supplemented Lysis Buffer#1 (1X) were dispensed into each well for 30 min at RT under gentle shaking. After cell lysis, 16 µL of lysates were transferred into a 384-well low volume white microplate and 4 µL of the HTRF Total Aurora A (#64AURATPEG/H), Phospho-Aurora A (Thr288), Total Aurora B (#64AURBTPEG/H), or Phospho-Aurora B (Thr232) (#64AURBT2PEG/H) detection antibodies were added. The HTRF signal was recorded after an overnight incubation.
The results indicate an inhibitor dose-dependent phosphorylation decrease with the expected selectivity behavior [2,3,4,5]:
The Aurora A and Aurora B protein expression levels remain constant (data not shown).
[2] : Manfredi et al. PNAS,2006
[3] : Sells et al. ACS Med.Chem.Lett.,2015
[4] : Tyler et al. Cell cycle,2007
[5] : Qi et al. Leuk Res,2013

Specificity and selectivity of HTRF Phospho-Aurora A assay using gene silencing (SiRNA)
HeLa cells were treated with 25 nM of SMARTPool ON-TARGETplus siRNA (Horizon) specifically targeting Aurora A (#L-003545-01-0005), Aurora B (# L-003326-00-0005), and Aurora C (#L-019573-00-0005), or with a non-targeting siRNA (# D-001810-10-20, included as control), in a 96-well plate (25,000 cells/well) under 100 µL for 24H. After cell culture medium removal, cells were stimulated by adding 300 nM Nocodazole in complete cell culture medium for an additional 24h-incubation at 37°C, 5% CO2. After medium removal, cells were lysed with 50 µL lysis buffer #1 (1X) for 30 min at RT under gentle shaking, and 16 µL of lysates were transferred into a low volume white microplate before the addition of 4 µL of premixed HTRF Phospho-Aurora A (Thr288) detection antibodies. The HTRF signal was recorded after an overnight incubation at RT. Cell treatment with Aurora A siRNA led to a significant downregulation of Aurora A, with a 57% signal decrease compared to the cells transfected with the non-targeting siRNA.
Despite high homology between the 3 family proteins, no decrease in signal was observed for cells treated with Aurora B or Aurora C siRNA, demonstrating the specificity and selectivity of the kit.

Validation on various Human cell lines
Immortalized human cell lines HepG2 (liver cancer), HeLa (Cervix cancer), HEK293, and HCT116 (Colon Cancer) were plated in 96-well culture plates at a density of 25,000 or 100,000 cells /well and incubated for 24 hours at 37°C, 5% CO2. After cell culture medium removal, cells were stimulated by adding 300 nM Nocodazole in complete cell culture medium for 20H at 37°C, 5% CO2. Cells were lysed after medium removal with 50 µL of supplemented lysis buffer #1 (1X) for 30 min at RT under gentle shaking.
The phosphorylated Aurora A protein expression level was assessed with the HTRF Phospho-Aurora A (Thr288) kit. Briefly, 16 µL of cell lysate were transferred into a low volume white microplate followed by 4 µL of premixed HTRF detection reagents. The HTRF signal was recorded after an overnight incubation at RT.
Phosphorylated Aurora A (Thr288) protein is well-detected in all the tested human cell lines at different levels. For a determined cellular model, cell density optimization is mandatory to be within the dynamic range of the kit. Note that HepG2, which expresses higher Aurora A protein levels, must be used at a lower cell density to be within the dynamic range (25,000 cells/well or below) compared to HeLa, HEK293, or HCT116 ( 25,000 to 100,000 cells/well).
The HTRF Phospho-Aurora A (Thr288) assay efficiently detects endogenous phosphorylated Aurora A protein in various human cellular models expressing different levels of the protein.

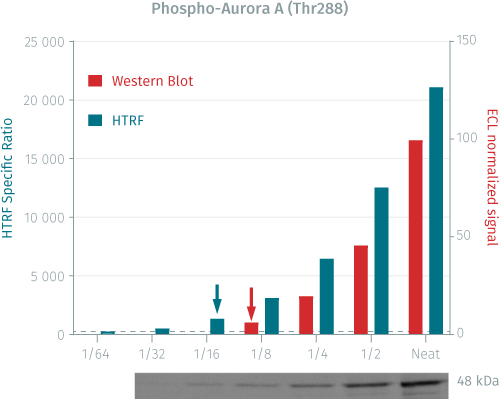
HTRF Phospho-Aurora A (Thr288) assay compared to Western Blot
HeLa cells were cultured in a T175 flask in complete culture medium at 37°C, 5% CO2. After 24H incubation, the cells were first stimulated with Nocodazole 300 nM for 20H, 37°C, 5% CO2, then lysed with 3 mL of supplemented lysis buffer #1 (1X) for 30 minutes at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer, and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of Phospho- Aurora A (Thr288) detection reagents. Equal amounts of lysates were used for a side by side comparison between HTRF and Western Blot.
A side by side comparison of Western Blot and HTRF demonstrates that the HTRF assay is 2-fold more sensitive than the Western Blot, at least under these experimental conditions
Resources
Are you looking for resources, click on the resource type to explore further.
This guide provides you an overview of HTRF applications in several therapeutic areas.
Loading...


Recently viewed


How can we help you?
We are here to answer your questions.






























