
HTRF Human Total ATF4 Detection Kit, 500 Assay Points

HTRF Human Total ATF4 Detection Kit, 500 Assay Points




This HTRF kit enables the cell-based quantitative detection of Total ATF4.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
This HTRF kit enables the cell-based quantitative detection of Total ATF4.


HTRF Human Total ATF4 Detection Kit, 500 Assay Points


HTRF Human Total ATF4 Detection Kit, 500 Assay Points


Product information
Overview
ATF4, a pivotal transcription factor in the EIF2a/ATF-4 signaling pathway, serves as the primary effector of the integrated stress response (ISR) triggered by PERK, HRI, GCN2, or PKR kinases. With a leucine Zipper domain (bZIP), ATF4 facilitates interactions with numerous proteins, acting as dimerization partners. Its multifaceted role extends across various tissues, significantly impacting obesity, glucose homeostasis, energy expenditure, and neural plasticity regulation.
As a stress-induced bZIP transcription factor, ATF4 binds to the cAMP response element, marking its involvement in the ISR, which encompasses responses to amino acid and glucose depletion, ER stress, and oxidative stress. This response entails the phosphorylation of eIF2alpha by various kinases, leading to global mRNA translation attenuation and ATF4 translation upregulation. Depending on post-translational modifications and binding partners, ATF4 can either promote pro-survival or apoptosis.
Additionally, ATF4's stability is subject to regulation through polyubiquitinylation, which mediates its proteasomal degradation.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Buffer/Solvent |
Lysis Buffer 4
|
| Detection Modality |
HTRF
|
| Host Species |
Human
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target |
ATF4
|
| Target Class |
Phosphoproteins
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Neuroscience
Oncology
|
| Unit Size |
500 assay points
|
How it works
Total ATF4 assay principle
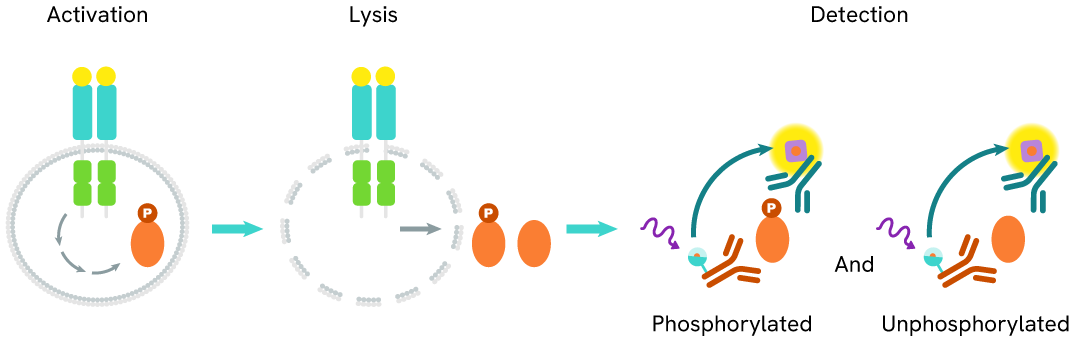
The Total ATF4 assay quantifies the expression level of ATF4 in a cell lysate. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Total ATF4 assay uses two labeled antibodies, one coupled to a donor fluorophore and the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In the presence of ATF4 in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the protein's expression under a no-wash assay format.

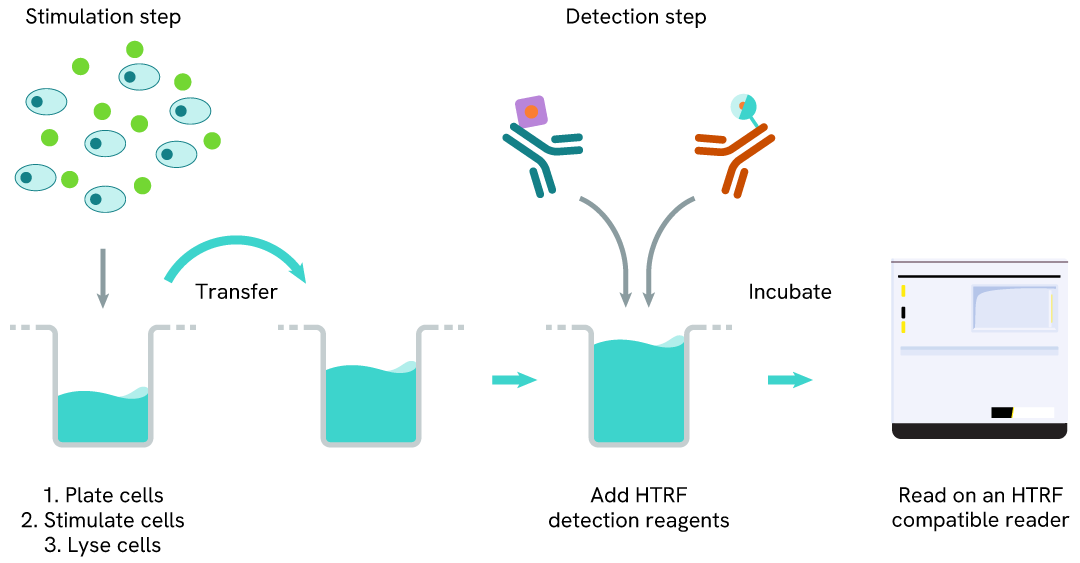
Total ATF4 two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of HTRF Total ATF4 detection reagents. This protocol allows for the cells' viability and confluence to be monitored.
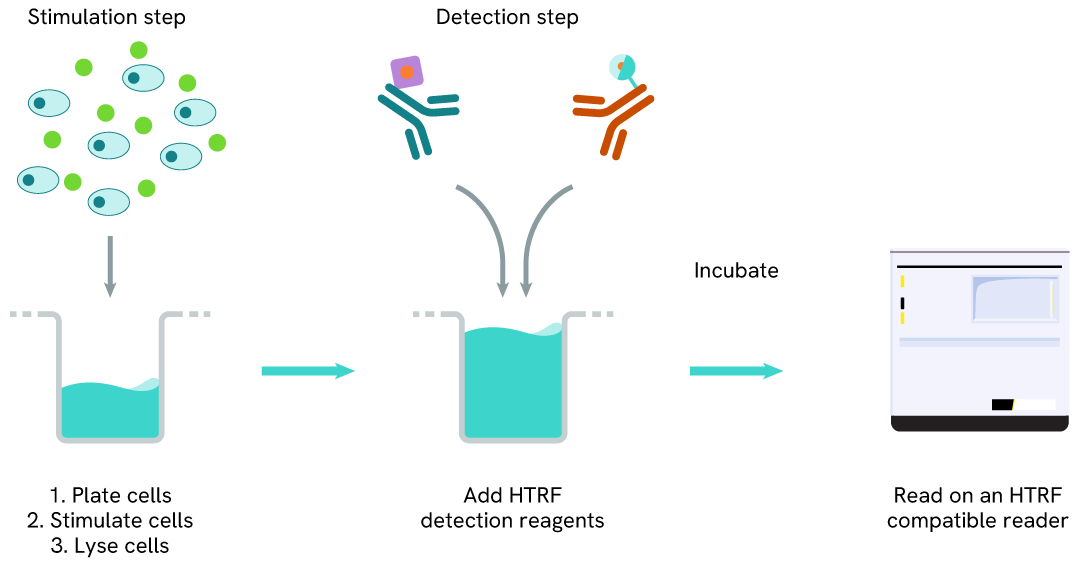
Total ATF4 one-plate assay protocol
Detection of Total ATF4 with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol facilitates miniaturization while maintaining robust HTRF quality.
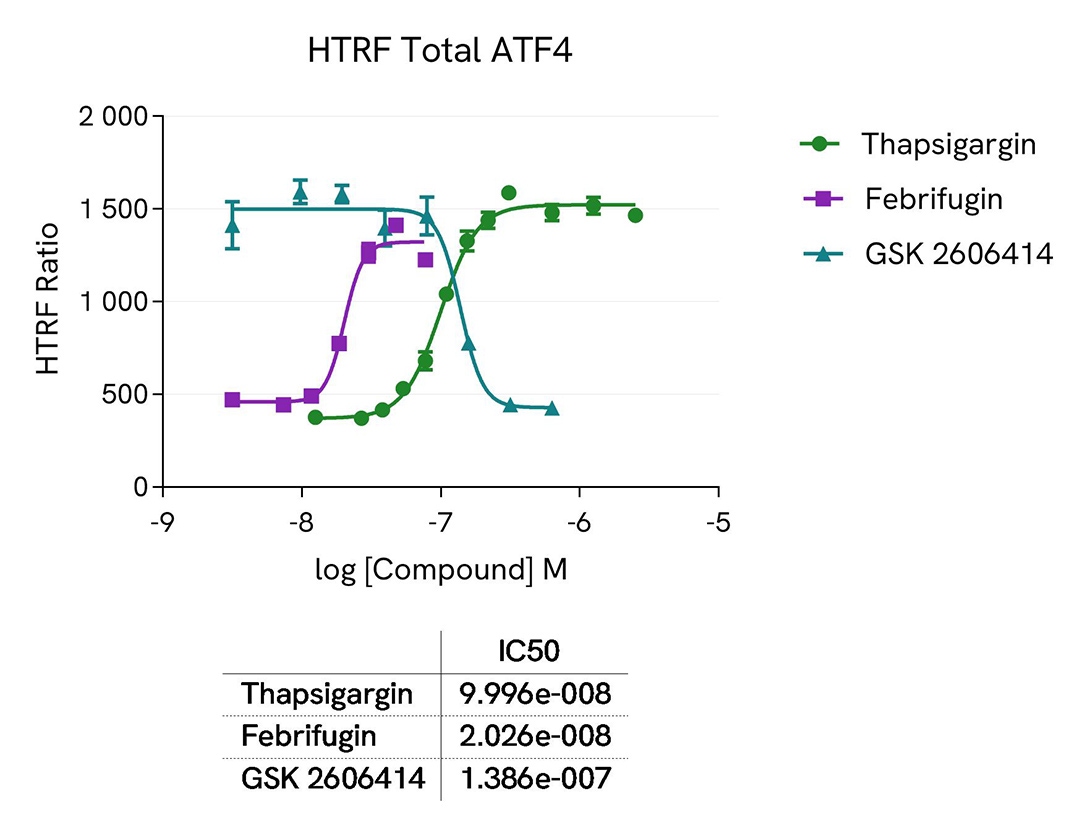
Assay validation
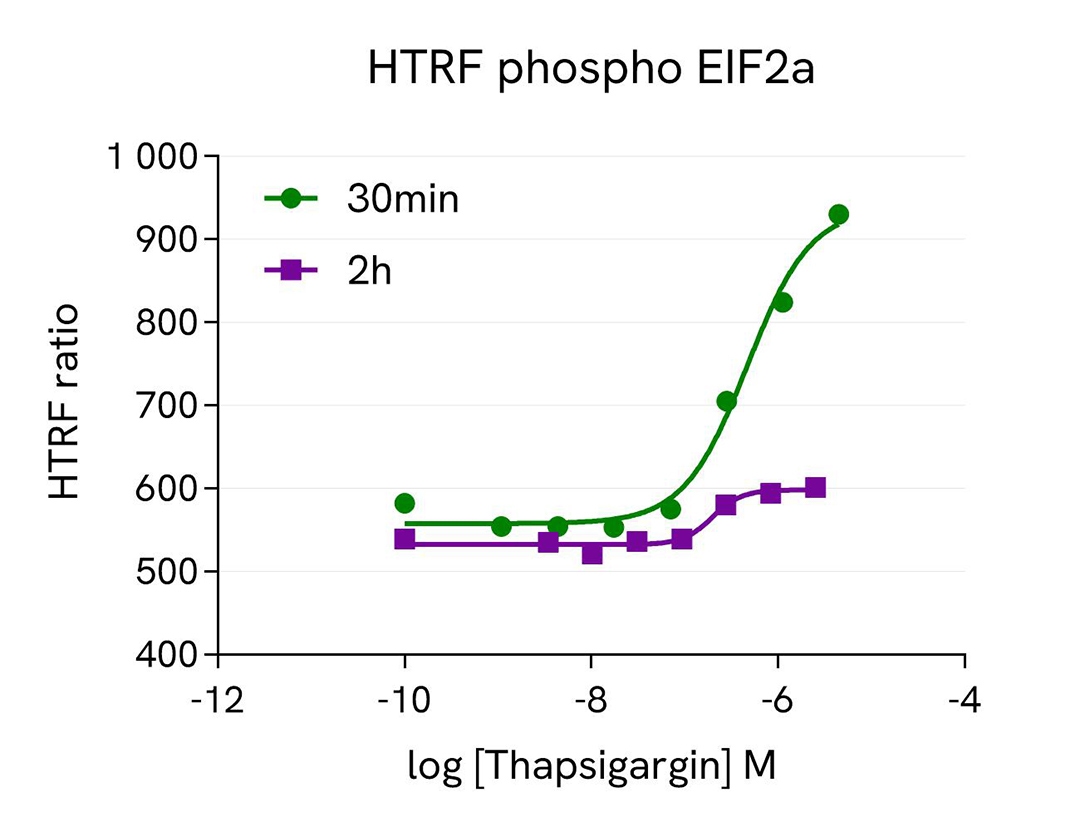
ATF4 expression dynamics in Hep-G2 cells under various treatment conditions
Hep-G2 cells were seeded in two 96-well culture-treated plates (50,000 cells per well) in a complete culture medium and incubated overnight at 37°C with 5% CO2. The cells were then treated with varying concentrations of the following compounds: Febrifugin (5 hours), Thapsigargin (30 minutes and 2 hours), and GSK2606414 (1 hour) on Thapsigargin-stimulated cells (200 nM, 2 hours).
Following treatment, the cells were lysed with 50 µL of supplemented lysis buffer #4 (1x) for 30 minutes at room temperature with gentle shaking. For detection, 16 µL of the cell lysate were transferred into a 384-well low-volume white microplate, and 4 µL of the HTRF Total ATF4 or HTRF phospho EIF2a detection reagents were added. The HTRF signal was recorded after 4 hours or overnight incubation for the Total ATF4 and phospho EIF2a assays, respectively.
As anticipated, the results showed that Thapsigargin and Febrifugin treatments increased ATF4 expression, whereas the GSK2606414 compound led to ATF4 downregulation. The effect of Thapsigargin on ATF4 expression was more pronounced after 2 hours of incubation, while a 30-minute treatment was optimal for the upstream EIF2a activation.
Proteasome inhibition enhances ATF4 expression in HEK293 cells
HEK293 cells were plated in a 96-well culture-treated plate at a density of 50,000 cells per well in complete culture medium and incubated overnight at 37°C in a 5% CO2 atmosphere. The cells were then treated with increasing doses of Epoxomycin for 4 hours.
After treatment, the cells were lysed with 50 µL of supplemented lysis buffer #4 (1x) for 30 minutes at room temperature with gentle shaking. For detection, 16 µL of cell lysate were transferred into a 384-well low-volume white microplate, and 4 µL of the HTRF Total-ATF4 detection reagents were added. The HTRF signal was measured after 4 hours of incubation.
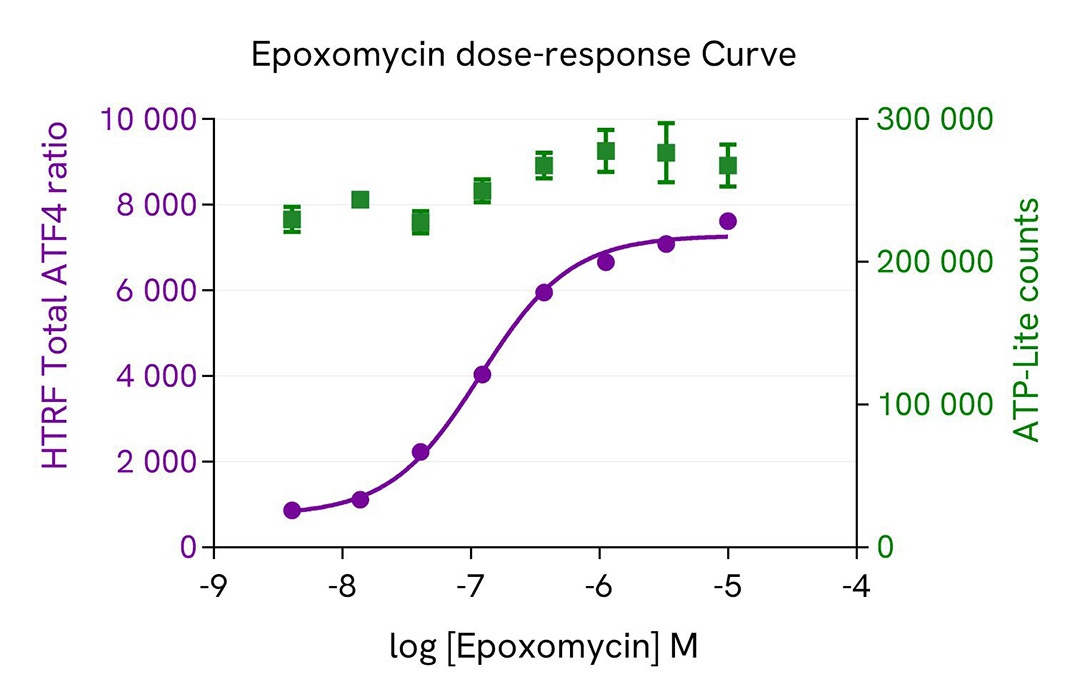
Additionally, cell viability was assessed by transferring 5 µL of the same lysate into an HTRF 96-well low-volume white plate (Cat # 66PL96005/025/100) and then adding 25 µL of ATPlite 1step detection reagent (ATPlite Luminescence Assay System, #6016943). The luminescence signal was measured after a 10-minute incubation in the dark.
This results evidence that proteasome inhibition mediated ATF4 protein stabilization which resulted in increased ATF4 expression level with increasing concentrations of epoxomycin. No cytoxicity effect was observed using the ATPlite Assay.
Validation of HTRF Total ATF4 assay specificity
HAP1 and HAP1 ATF4 knockout (KO) cells, were cultured in a T175 flask with complete culture medium for 24 hours at 37°C in a 5% CO2 environment. After incubation, the cells were lysed with 3 mL of supplemented lysis buffer #4 (1x) for 30 minutes at room temperature with gentle shaking. Subsequently, 16 µL of the lysate were transferred into a 384-well low-volume white microplate, followed by the addition of 4 µL of the HTRF total ATF4 detection reagents. The HTRF signal was recorded after a 4-hour incubation at room temperature.
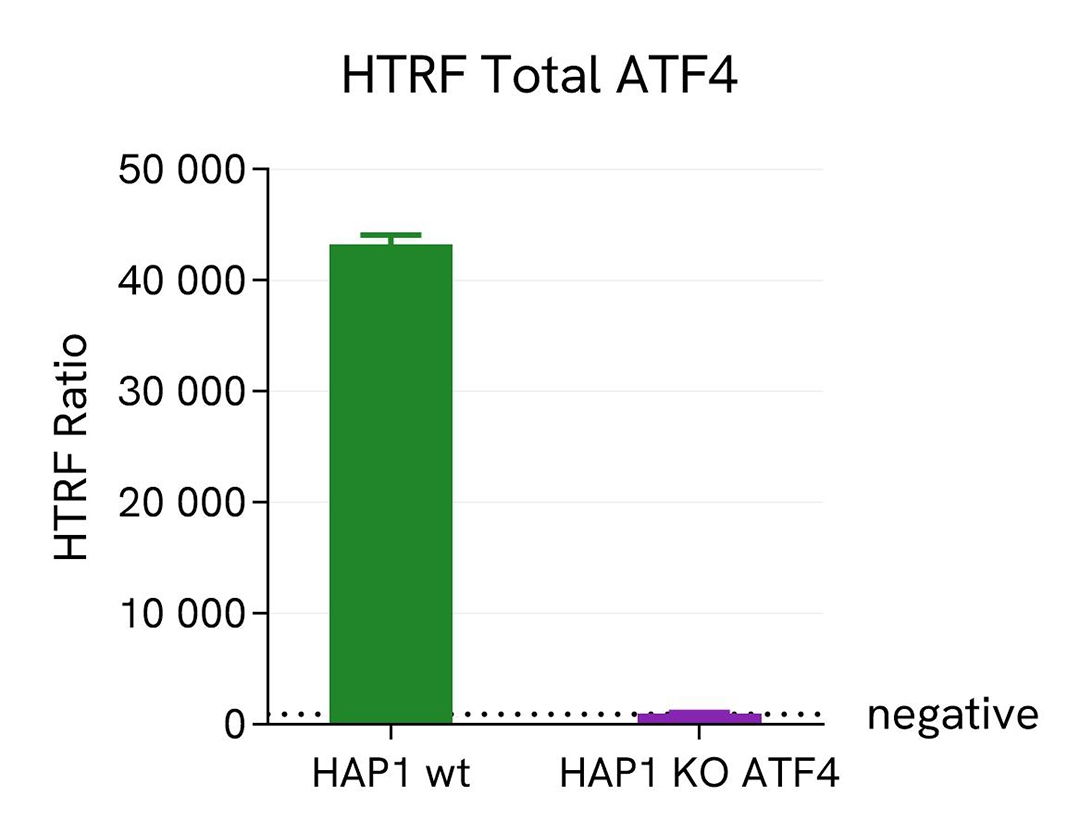
As shown on the graph, no signal was detected for the HAP1 cells with KO ATF4, whereas a signal was detected in the wild-type HAP1 cells, thus confirming the assay's specificity for ATF4 protein.
HTRF ATF4 protein detection in human cell lines
Human adherent cells (HEK293 & HepG2) and suspension cells (THP1) were seeded at densities of 100,000 and 300,000 cells per well, respectively, in a 96-well microplate. Following a 24-hour incubation period, the cells were treated with 700 nM of thapsigargin for 2 hours at 37°C in a 5% CO2 environment. Subsequently, the cells were lysed for 30 minutes using supplemented lysis buffer #4, following the respective protocols for adherent or suspended cells, at room temperature with gentle shaking.
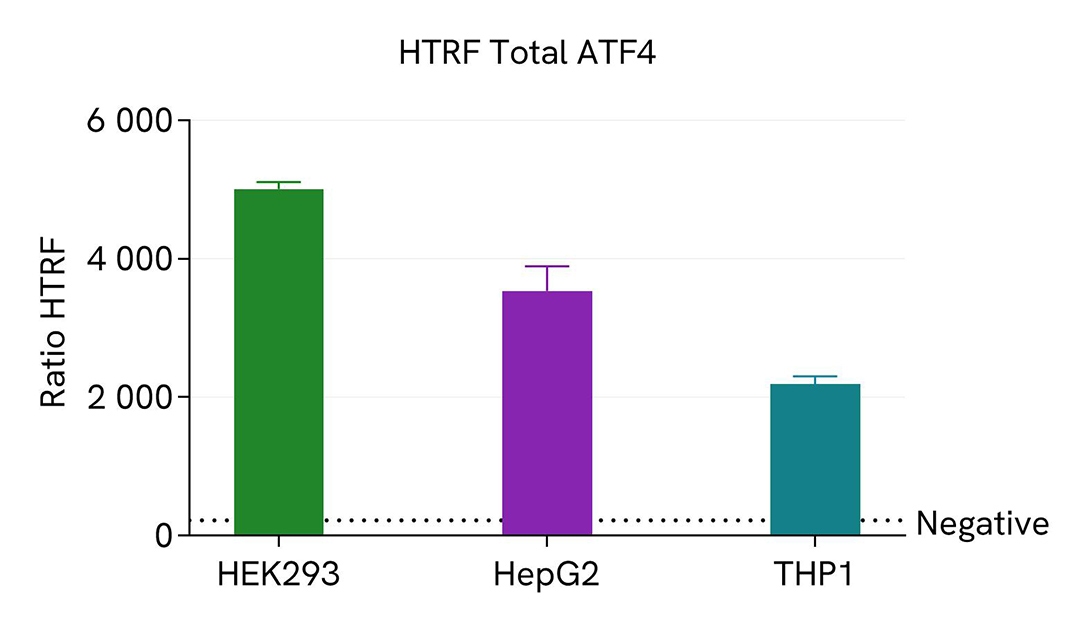
Next, 16 µL of the lysate were transferred into a 384-well low-volume white microplate, followed by the addition of 4 µL of the HTRF Total ATF4 detection reagents. The HTRF signal was recorded after a 4-hour incubation period.
The HTRF Total ATF4 assay demonstrated detection of ATF4 protein in various cellular models expressing different levels of the protein.
HTRF Total ATF4 assay compared to western blot
HEK293 cells were cultured in a T175 flask in complete culture medium at 37°C - 5% CO2 until 80% confluence. The cells were then treated with 10 µM of MG132 for 5h. Then the cells were lysed with 3 mL of supplemented lysis buffer # 4 (1x) for 30 minutes at RT under gentle shaking.
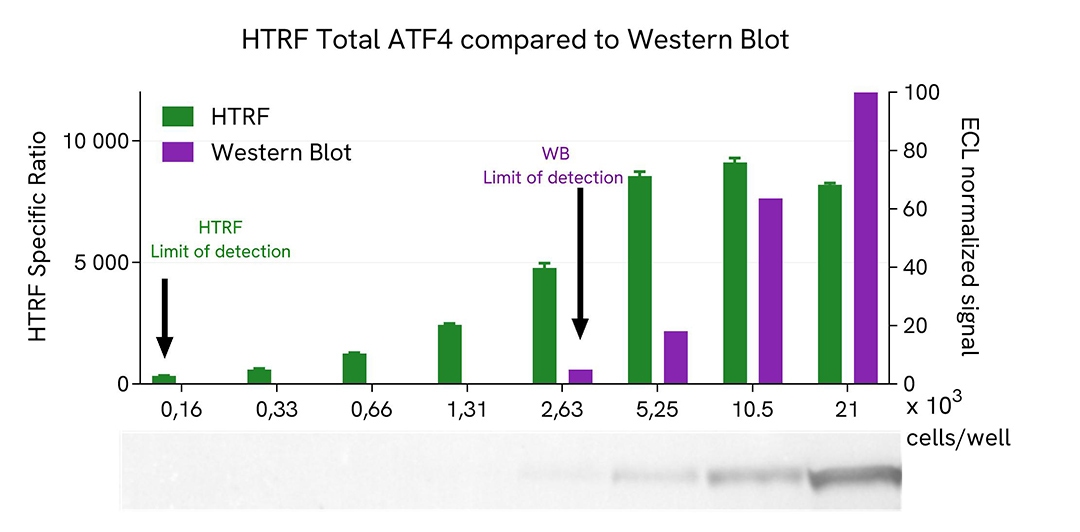
Serial dilutions of the cell lysate were performed using supplemented lysis buffer (1x), and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF Total ATF4 detection reagents. Equal amounts of lysates were used for a side-by-side comparison between HTRF and Western Blot.
Therefore, in these conditions, the results shows that the HTRF Total ATF4 assay was 16 times more sensitive than the Western Blot technique.
Simplified pathway
ATF4 signaling pathway
ATF4, a transcription factor, is a key mediator in the integrated stress response (ISR) pathway. Under stress conditions such as nutrient deprivation, ATF4 expression is upregulated through the phosphorylation of eIF2α. Once activated, ATF4 translocates to the nucleus and binds to specific DNA sequences known as C/EBP-ATF response elements (CARE). This binding leads to the transcriptional regulation of genes involved in various cellular processes, including amino acid metabolism, redox homeostasis, and apoptosis. Additionally, ATF4 interacts with other transcription factors and co-regulators to fine-tune gene expression in response to stress.
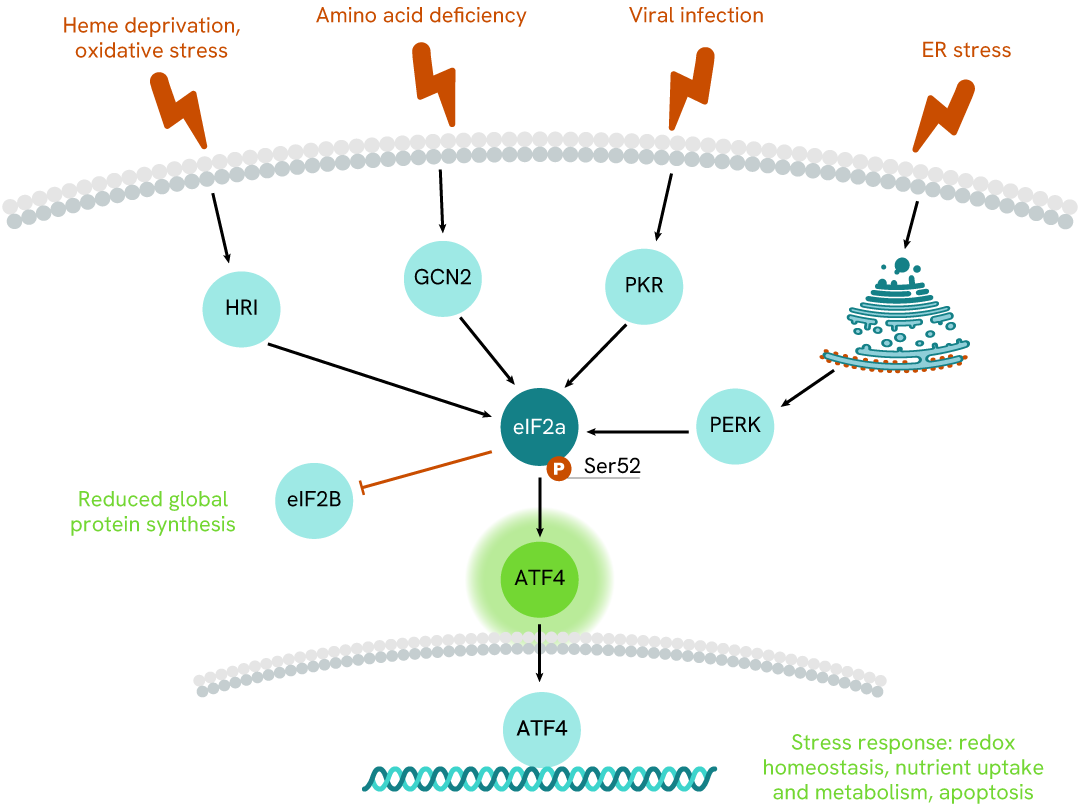
Dysregulation of ATF4 signaling has been implicated in various diseases, including cancer, neurodegenerative disorders, and metabolic syndromes.
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
SDS, COAs, Manuals and more
Are you looking for technical documents related to the product? We have categorized them in dedicated sections below. Explore now.
- LanguageEnglishCountryUnited States
- LanguageFrenchCountryFrance
- LanguageGermanCountryGermany
- Lot Number02ALot DateApril 14, 2027
- Lot Number01ALot DateApril 3, 2026
- Resource TypeManualLanguageEnglishCountry-


How can we help you?
We are here to answer your questions.






























