
AlphaLISA SureFire Ultra Human and Mouse Cleaved PARP1 D214 Detection Kit, 50,000 Assay Points
AlphaLISA SureFire Ultra Human and Mouse Cleaved PARP1 D214 Detection Kit, 50,000 Assay Points


The AlphaLISA™ SureFire® Ultra™ Human and Mouse Cleaved PARP1 D214 assay is a sandwich immunoassay for quantitative detection of cleaved PARP1 D214 in cellular lysates using Alpha Technology.
For research use only. Not for use in diagnostic procedures. All products to be used in accordance with applicable laws and regulations including without limitation, consumption and disposal requirements under European REACH regulations (EC 1907/2006).
| Feature | Specification |
|---|---|
| Application | Protein Quantification |
The AlphaLISA™ SureFire® Ultra™ Human and Mouse Cleaved PARP1 D214 assay is a sandwich immunoassay for quantitative detection of cleaved PARP1 D214 in cellular lysates using Alpha Technology.
For research use only. Not for use in diagnostic procedures. All products to be used in accordance with applicable laws and regulations including without limitation, consumption and disposal requirements under European REACH regulations (EC 1907/2006).


AlphaLISA SureFire Ultra Human and Mouse Cleaved PARP1 D214 Detection Kit, 50,000 Assay Points


AlphaLISA SureFire Ultra Human and Mouse Cleaved PARP1 D214 Detection Kit, 50,000 Assay Points


Product information
Overview
Cleaved Poly (ADP-ribose) polymerase 1 (PARP1) is a marker of apoptosis, a form of programmed cell death. PARP1 is a DNA repair enzyme that becomes activated in response to DNA damage. During apoptosis, PARP1 is cleaved by caspases, specifically at Asp214, rendering it inactive and preventing DNA repair, which facilitates the apoptotic process. Cleaved PARP1 serves as an important biomarker for apoptosis in cancer research and therapeutic assessment.
The AlphaLISA SureFire Ultra Human and Mouse Cleaved PARP1 D214 Detection Kit is a sandwich immunoassay for the quantitative detection of Cleaved PARP1 D214 in cellular lysates, using Alpha Technology.
Formats:
- The HV (high volume) kit contains reagents to run 100 wells in 96-well format, using a 60 μL reaction volume.
- The 500-point kit contains enough reagents to run 500 wells in 384-well format, using a 20 μL reaction volume.
- The 10,000-point kit contains enough reagents to run 10,000 wells in 384-well format, using a 20 μL reaction volume.
- The 50,000-point kit contains enough reagents to run 50,000 wells in 384-well format, using a 20 μL reaction volume.
AlphaLISA SureFire Ultra kits are compatible with:
- Cell and tissue lysates
- Antibody modulators
- Biotherapeutic antibodies
Alpha SureFire Ultra kits can be used for:
- Cellular kinase assays
- Receptor activation studies
- Screening
Specifications
| Application |
Protein Quantification
|
|---|---|
| Automation Compatible |
Yes
|
| Brand |
AlphaLISA SureFire Ultra
|
| Detection Modality |
Alpha
|
| Host Species |
Human
Mouse
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Shipping Conditions |
Shipped in Blue Ice
|
| Target |
PARP1 D214
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
Alpha
|
| Therapeutic Area |
Oncology
|
| Unit Size |
50,000 Assay Points
|
How it works
Total-AlphaLISA SureFire Ultra assay principle
The Total-AlphaLISA SureFire Ultra assay measures the expression level of a protein target in a cell lysate.
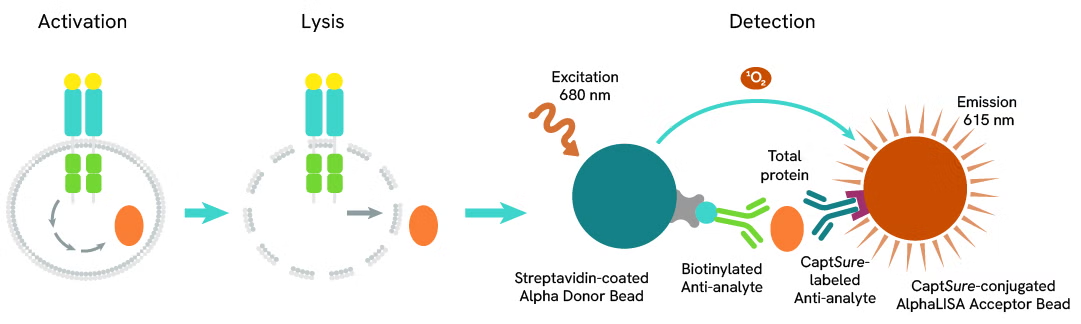
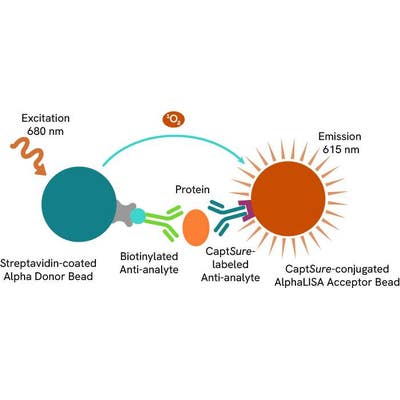
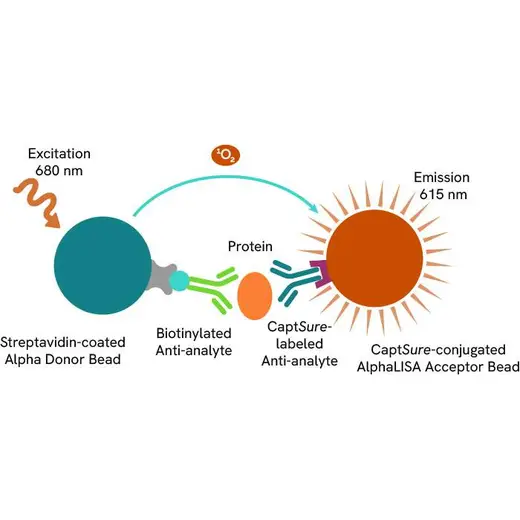
The Total-AlphaLISA SureFire Ultra assay uses two antibodies which recognize two different distal epitopes on the targeted protein. AlphaLISA assays require two bead types: Acceptor and Donor beads. Acceptor beads are coated with a proprietary CaptSure™ agent to specifically immobilize the assay specific antibody, labeled with a CaptSure™ tag. Donor beads are coated with streptavidin to capture one of the detection antibodies, which is biotinylated. In the presence of targeted protein, the two antibodies bring the Donor and Acceptor beads in close proximity whereby the singlet oxygen transfers energy to excite the Acceptor bead, allowing the generation of a luminescent Alpha signal. The amount of light emission is directly proportional to the quantity of protein present in the sample.

Total-AlphaLISA SureFire Ultra two-plate assay protocol
The two-plate protocol involves culturing and treating the cells in a 96-well plate before lysis, then transferring lysates into a 384-well OptiPlate™ plate before the addition of Total-AlphaLISA SureFire Ultra detection reagents. This protocol permits the cells' viability and confluence to be monitored. In addition, lysates from a single well can be used to measure multiple targets.
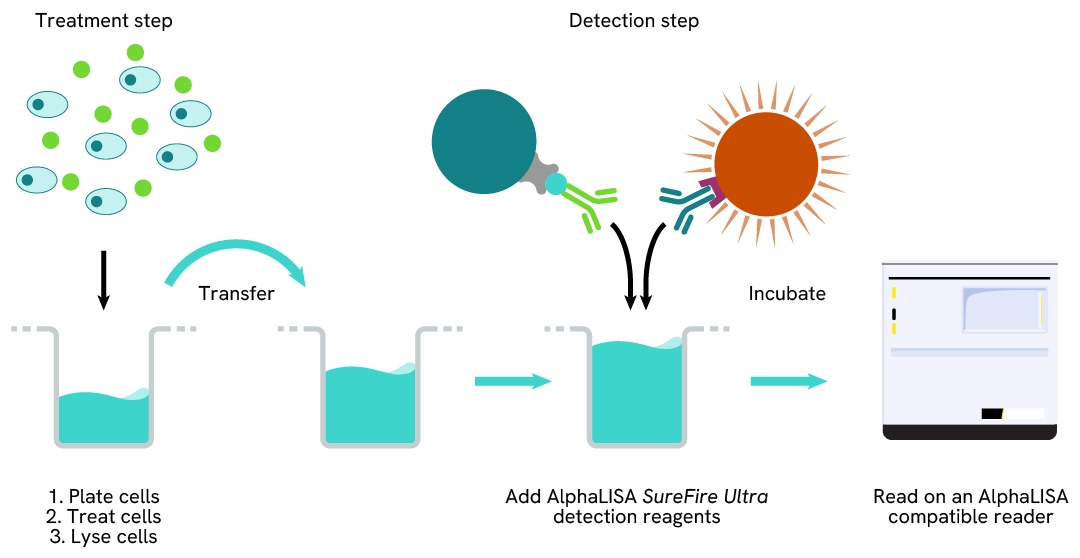
Total-AlphaLISA SureFire Ultra one-plate assay protocol
Detection of Total target protein with AlphaLISA SureFire Ultra reagents can be performed in a single plate used for culturing, treatment, and lysis. No washing steps are required. This HTS designed protocol allows for miniaturization while maintaining AlphaLISA SureFire Ultra quality.
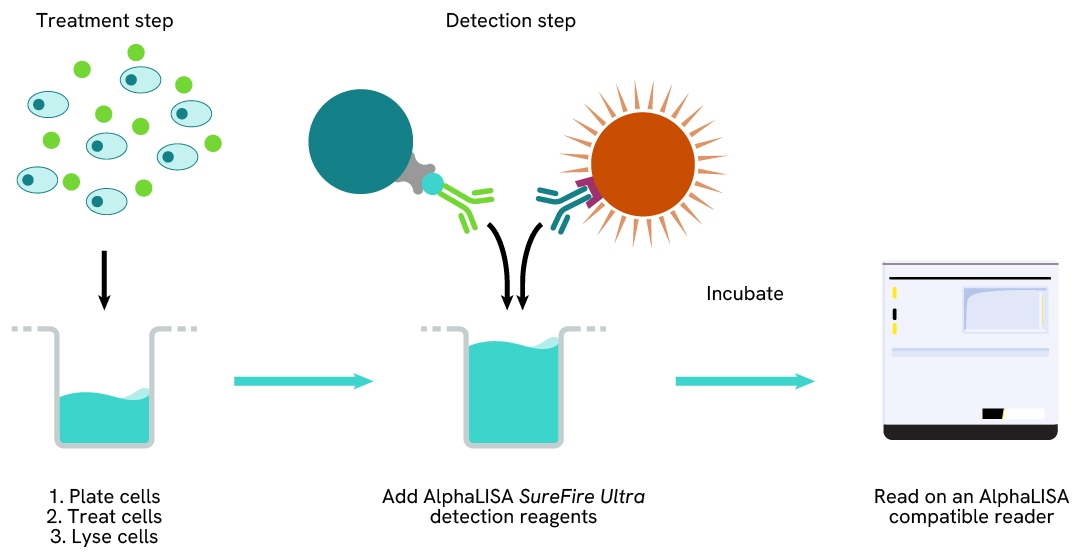
Assay validation
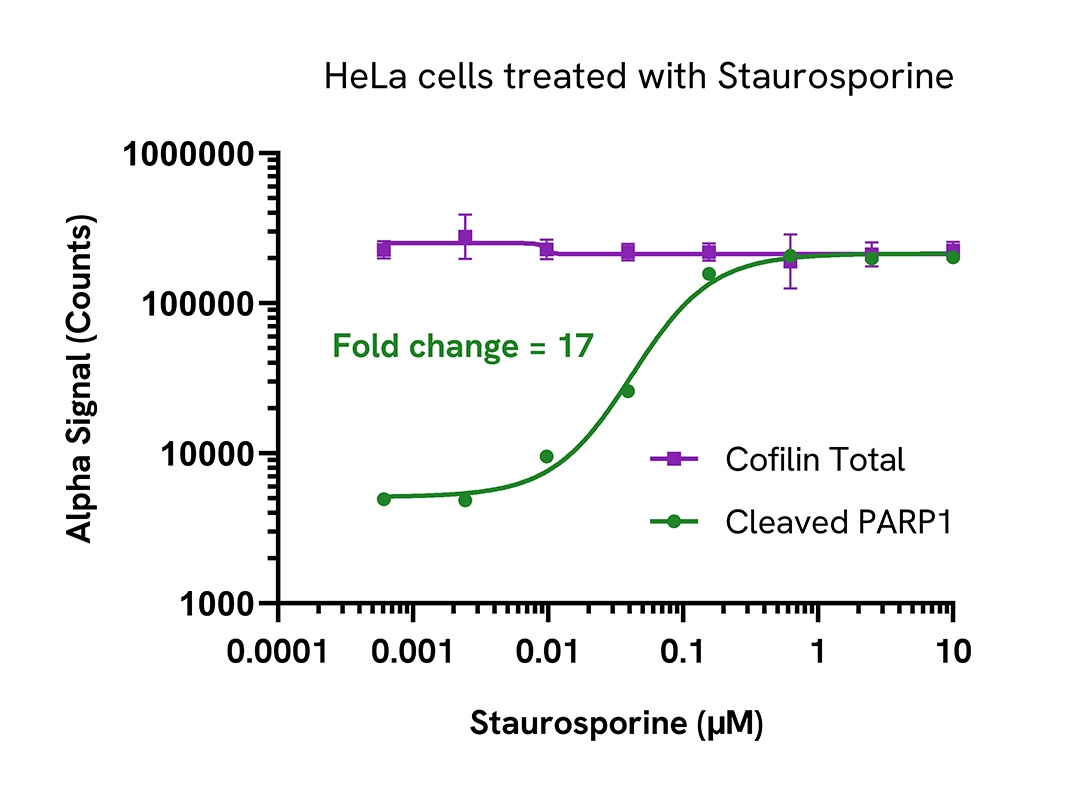
Activation of cleaved PARP1 (Asp214) in Staurosporine treated human cells
HeLa cells were seeded in a 96-well plate (40,000 cells/well) in complete medium and incubated overnight at 37°C, 5% CO2. The cells were treated with increasing concentrations of Staurosporine for 4 hours in serum free medium.
After treatment, the cells were lysed with 100 µL of Lysis Buffer for 10 minutes at RT with shaking (350 rpm). Cleaved PARP1 (Asp214) and Total Cofilin were evaluated using respective AlphaLISA SureFire Ultra assays. For the detection step, 10 µL of cell lysate (approximately 4,000 cells or 800 cells for Total Cofilin) was transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor mix and incubated for 1 hour at RT. Finally, 5 µL of Donor mix was then added to each well and incubated for 1 hour at RT in the dark. The plate was read on an Envision using standard AlphaLISA settings.
As expected, Staurosporine triggered a dose-dependent increase in the levels of Cleaved PARP1 (Asp214) while Total Cofilin levels remained unchanged.
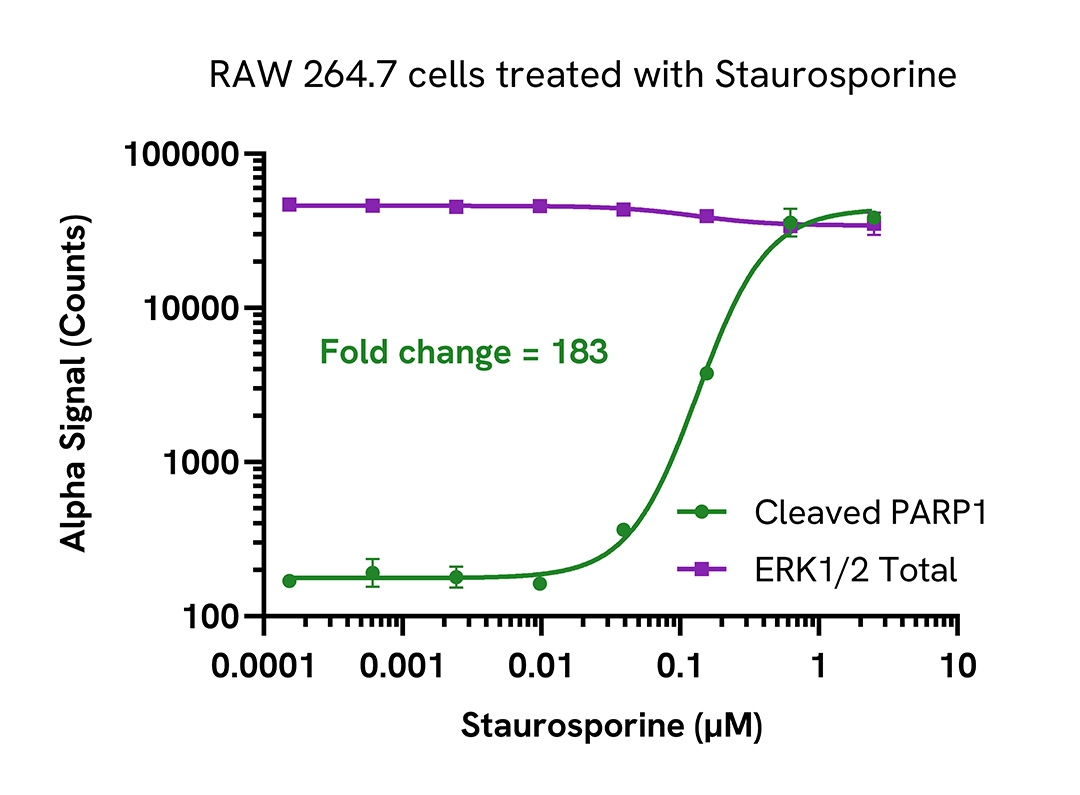
Activation of cleaved PARP1 (Asp214) in Staurosporine treated mouse cells
RAW 264.7 cells were seeded in a 96-well plate (40,000 cells/well) in complete medium and incubated overnight at 37°C, 5% CO2. The cells were treated with increasing concentrations of Staurosporine for 4 hours in serum free medium.
After treatment, the cells were lysed with 100 µL of Lysis Buffer for 10 minutes at RT with shaking (350 rpm). Cleaved PARP1 (Asp214) and Total ERK1/2 were evaluated using respective AlphaLISA SureFire Ultra assays. For the detection step, 10 µL of cell lysate (approximately 4,000 cells) was transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor mix and incubated for 1 hour at RT. Finally, 5 µL of Donor mix was then added to each well and incubated for 1 hour at RT in the dark. The plate was read on an Envision using standard AlphaLISA settings.
As expected, Staurosporine triggered a dose-dependent increase in the levels of Cleaved PARP1 (Asp214) while Total ERK1/2 levels remained unchanged.
Inhibition of cleaved PARP1 (Asp214) in Olaparib treated cells
HeLa cells were seeded in a 96-well plate (40,000 cells/well) in complete medium, and incubated overnight at 37°C, 5% CO2. The cells were pretreated with 1 µM Staurosporine for 4 hours in serum free medium, followed by treatment with increasing concentrations of Olaparib for 10 minutes.
After treatment, the cells were lysed with 100 µL of Lysis Buffer for 10 minutes at RT with shaking (350 rpm). Cleaved PARP1 (Asp214) and Total Cofilin were evaluated using respective AlphaLISA SureFire Ultra assays.
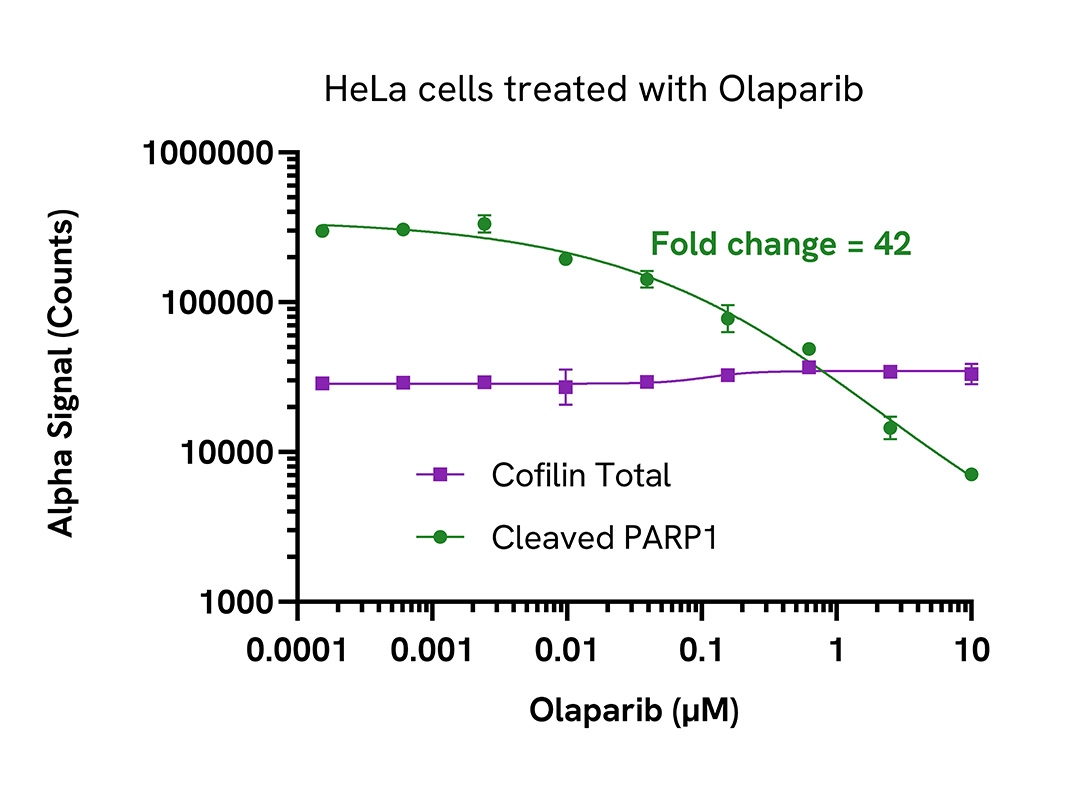
For the detection step, 10 µL of cell lysate (approximately 4,000 cells or 800 cells for Total Cofilin) was transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor mix and incubated for 1 hour at RT. Finally, 5 µL of Donor mix was then added to each well and incubated for 1 hour at RT in the dark. The plate was read on an Envision using standard AlphaLISA settings.
As expected, Olaparib, a well validated PARP1/2 inhibitor, triggered a dose-dependent inhibition of Cleaved PARP1 (Asp214) while Total Cofilin levels remain unchanged.
Assay specificity/selectivity
Specificity of cleaved PARP1 (Asp214) assay
Cleaved PARP1 (Asp214) protein levels were assessed in A549 wild type (WT) and A549 PARP1 knockout (KO) (Abcam, ab276094) cells. Both cell lines were grown to confluency in a T75 flask in medium at 37°C, 5% CO2. Each flask was treated with 1 µM Staurosporine for 4 hours in serum free medium and then lysed in 2 mL of Lysis Buffer for 10 minutes at RT with shaking (350 rpm). Lysates were then serially diluted in Lysis Buffer and evaluated for Cleaved PARP1 (Asp214) using the AlphaLISA SureFire Ultra kit.
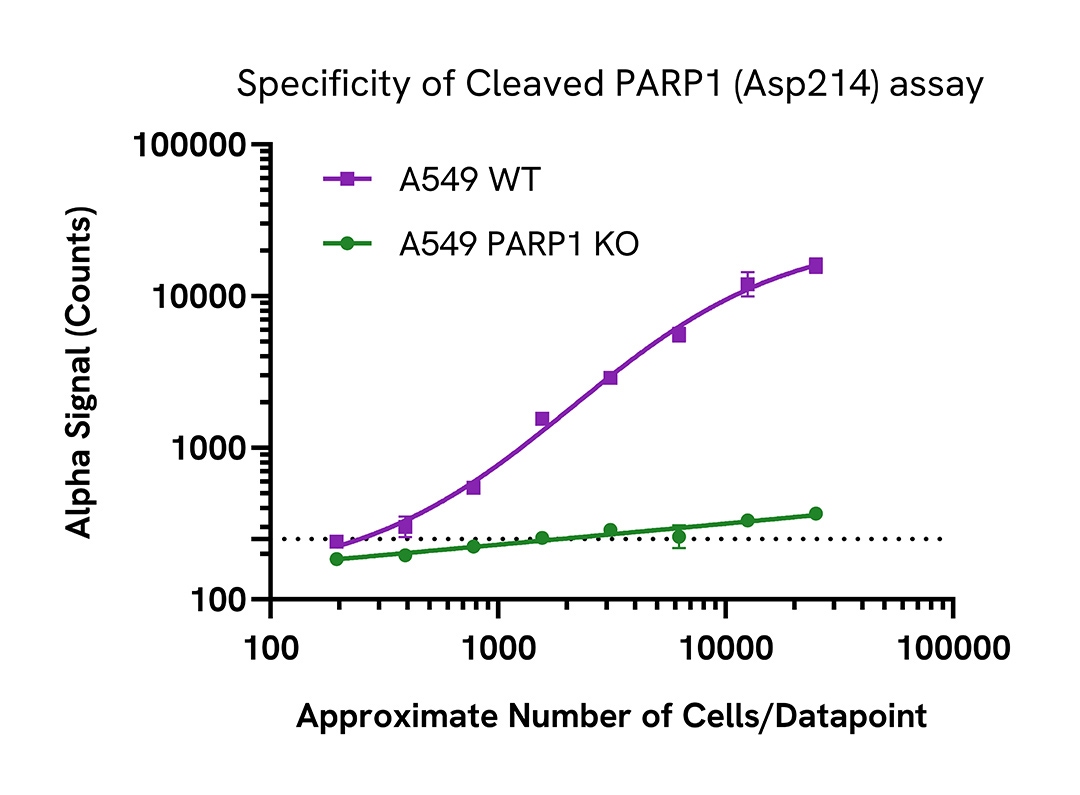
For the detection step, 10 µL of cell lysate was transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor Mix and incubated for 1 hour at RT. Finally, 5 µL of Donor Mix was added to each well and incubated for 1 hour at RT in the dark. The plate was read on an Envision using standard AlphaLISA settings.
As expected, Cleaved PARP1 (Asp214) was only detected in the WT A549 cells but not in the A549 PARP1 KO cell line, demonstrating assay specificity. Assay background is represented with a dotted line.
Resources
Are you looking for resources, click on the resource type to explore further.


How can we help you?
We are here to answer your questions.






























