High-grade serous ovarian cancer (HGSOC) is a lethal histotype of ovarian cancer (OC), accounting for approximately 70% of all OC cases and 80% of deaths (1). The 5-year survival rate for HGSOC is dismal at 30%, indicating an urgent need for improved treatments. HGSOC is characterized by a high degree of genetic instability, with amplification of CCNE1, a gene encoding Cyclin E1, occurring in roughly 15-20% of cases (2). CCNE1-amplified HGSOC cells require homologous recombination (HR) to repair collapsed replication forks, allowing them to proliferate and induce premature entry into the S-phase of the cell cycle.
The Role of Cyclins and CDKs in Cell Cycle Regulation:
Cyclins and cyclin-dependent kinases (CDKs) are essential cell cycle regulators, ensuring that cells progress through each phase in a tightly regulated manner (3). Cyclins are dynamic proteins that exhibit fluctuating levels throughout the cell cycle, binding to and activating CDKs at specific checkpoints. CDKs are serine/threonine kinases that, when activated by cyclins, phosphorylate target proteins to drive the cell cycle forward. Dysregulation of cyclin and CDK activity can lead to uncontrolled proliferation, a hallmark of cancer.

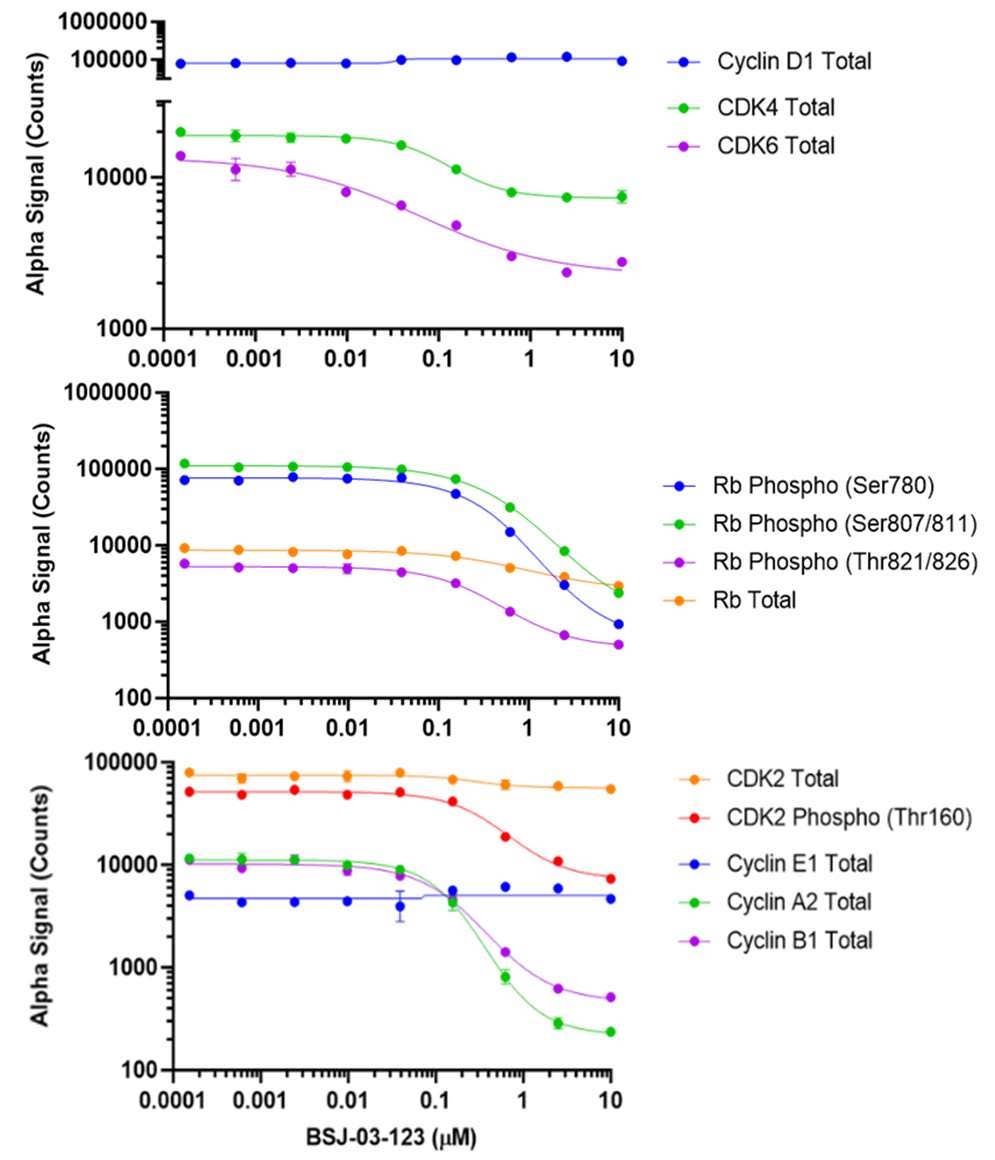
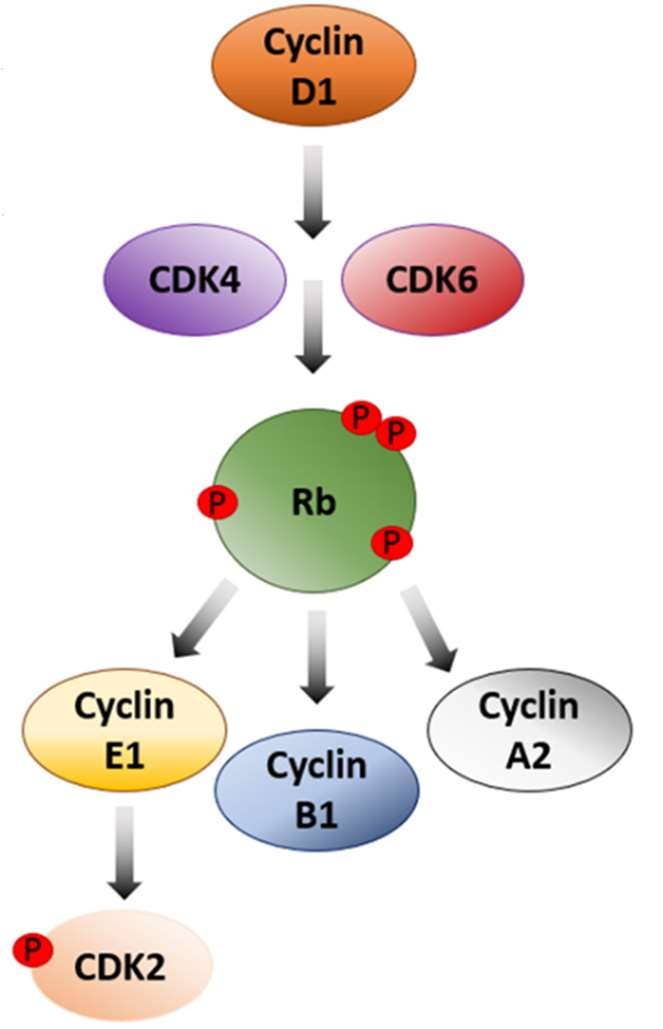
Figure 1. Regulatory Network of Cyclins and CDKs in the Cell Cycle and Application of AlphaLISA® SureFire® Ultra™ Technology*. Diagram illustrating the regulatory network involving cyclins and CDKs in the cell cycle and highlights the versatility of AlphaLISA SureFire Ultra technology for detecting protein levels at each phase. The cell cycle initiates with Cyclin D1 activating CDK4/6, promoting progression from G1 to S-phase. This Cyclin D1/CDK4/6 complex phosphorylates Retinoblastoma (Rb), a key tumor suppressor, leading to the release of E2F transcription factors necessary for DNA synthesis and S-phase entry. In its hypophosphorylated state, Rb inhibits E2F and halts progression. Cyclin E1 then activates CDK2, further advancing the G1/S-phase transition. During the M-phase, Cyclin A2 and Cyclin B1 bind to CDK2 and CDK1, respectively, to regulate mitotic events. To demonstrate the capabilities of AlphaLISA SureFire Ultra, a dose-response curve of cell cycle regulator protein levels is shown with MCF7 cell lysates treated with BSJ-03-123 PROTAC. AlphaLISA signal was measured using an EnVision 2105 Multimode Plate Reader. Appx. 2,000 cells/data point (8,000 for CDK6). *Note: AlphaLISA SureFire Ultra is a subset of AlphaLISA technology used for measuring cellular signaling cascade events
CDK2: A Key Player in HGSOC Replication
One critical CDK involved in the cell cycle is CDK2, the canonical partner of Cyclin E1, which plays a key role in HR by facilitating the G1/S-phase transition. The role of CDK2 in the context of CCNE1-amplification is an essential driver for repairing collapsed replication forks due to the replication stress caused by Cyclin E1 overexpression. CCNE1-amplified tumor cells exhibit intrinsic resistance to current treatments for HGSOC, including poly (ADP-ribose) polymerase (PARP) inhibitors and other chemotherapy agents (2). This resistance underscores a significant gap in our understanding and highlights the need for alternative therapeutic strategies.
In a study published in NAR Cancer by Brown et al., the role of CDK2 in repairing collapsed replication forks in CCNE1-amplified OC cells was explored by elucidating the underlying signaling pathways. Their study also demonstrates that inhibition of CDK2 with a selective inhibitor in CCNE-1 amplified OC cells results in sensitization to other DNA-damaging agents, revealing a new potential treatment strategy for patients with HGSOC. One critical CDK involved in the cell cycle is CDK2, the canonical partner of Cyclin E1, which plays a key role in HR by facilitating the G1/S-phase transition. The role of CDK2 in the context of CCNE1-amplification is an essential driver for repairing collapsed replication forks due to the replication stress caused by Cyclin E1 overexpression. CCNE1-amplified tumor cells exhibit intrinsic resistance to current treatments for HGSOC, including poly (ADP-ribose) polymerase (PARP) inhibitors and other chemotherapy agents (2). This resistance underscores a significant gap in our understanding and highlights the need for alternative therapeutic strategies.
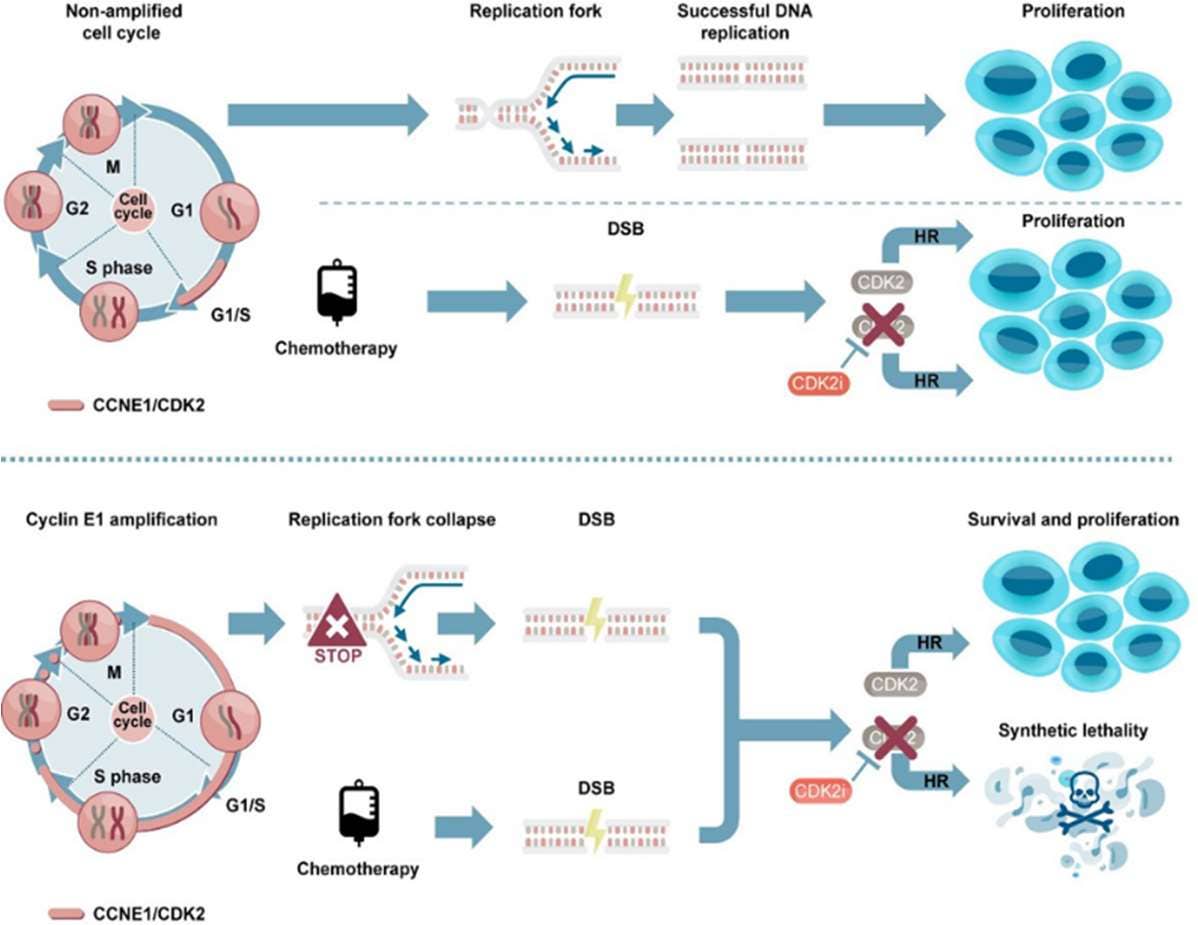
Figure 2. Graphical abstract from Brown et al. highlighting the role of CDK2 in repairing the replication fork to collapse seen in the Cyclin E1-amplified cell cycle, thus, facilitating cell survival and proliferation. By inhibiting CDK2, homologous replication is impaired, leading to synthetic lethality when combined with chemotherapy agents.
CCNE1-Amplified Cells Require CDK2 for CHK1 Signaling and HR
-
The Role of CDK2 in CHK1 Signaling and HR:
CHK1 (Checkpoint Kinase 1) is a key protein kinase involved in the cellular response to DNA damage and replication stress. It is activated in response to stalled replication forks or DNA damage during the S and G2 phases of the cell cycle. CHK1 maintains genomic stability by coordinating DNA repair mechanisms and arresting cell cycle progression to allow time for damage resolution. Specifically, CHK1 phosphorylates various substrates to inhibit cyclin-dependent kinases (CDKs) and other cell cycle regulators, thereby preventing premature progression into mitosis when DNA is damaged or replication is incomplete. In CCNE1-amplified OC cells, CDK2 is integral for effective CHK1 signaling and HR. The interaction between CDK2 and CHK1 ensures that DNA repair processes are efficiently executed, CDK2, in complex with Cyclin E1, facilitates the G1/S-phase transition and supports HR by enabling proper repair of replication fork collapses. The study utilized a selective CDK2 inhibitor, BLU1851, to examine the role of CDK2 in these processes.
-
Implications for CHK1 Signaling and Replication Stress:
The selective inhibition of CDK2 led to a marked suppression of CHK1 activation in CCNE1-amplified cells, as measured by decreased phosphorylation at S317. This highlights the crucial role of CDK2 in maintaining CHK1 signaling and replication fork stability under stress conditions.
-
Inhibition of CDK2:
Utilizing the potent and selective CDK2 inhibitor, BLU1851, the study employed AlphaLISA assays to examine the phosphorylation status of key substrates: Retinoblastoma protein 1 (Rb1) and lamin A/C, as proxies for CDK2 and CDK1 activities, respectively. The results demonstrated that BLU1851 significantly reduced the phosphorylation of Rb (Supplementary Figure S2F), confirming effective inhibition of CDK2 activity in CCNE1-amplified OVCAR-3 cells. In contrast, the phosphorylation levels of lamin A/C remained largely unaffected (Supplementary Figure S2G). This result highlights the specificity of BLU1851 for CDK2, with minimal off-target effects on CDK1, thereby confirming the selective inhibition of CDK2 to further confirm its role in CHK1 signaling and HR.
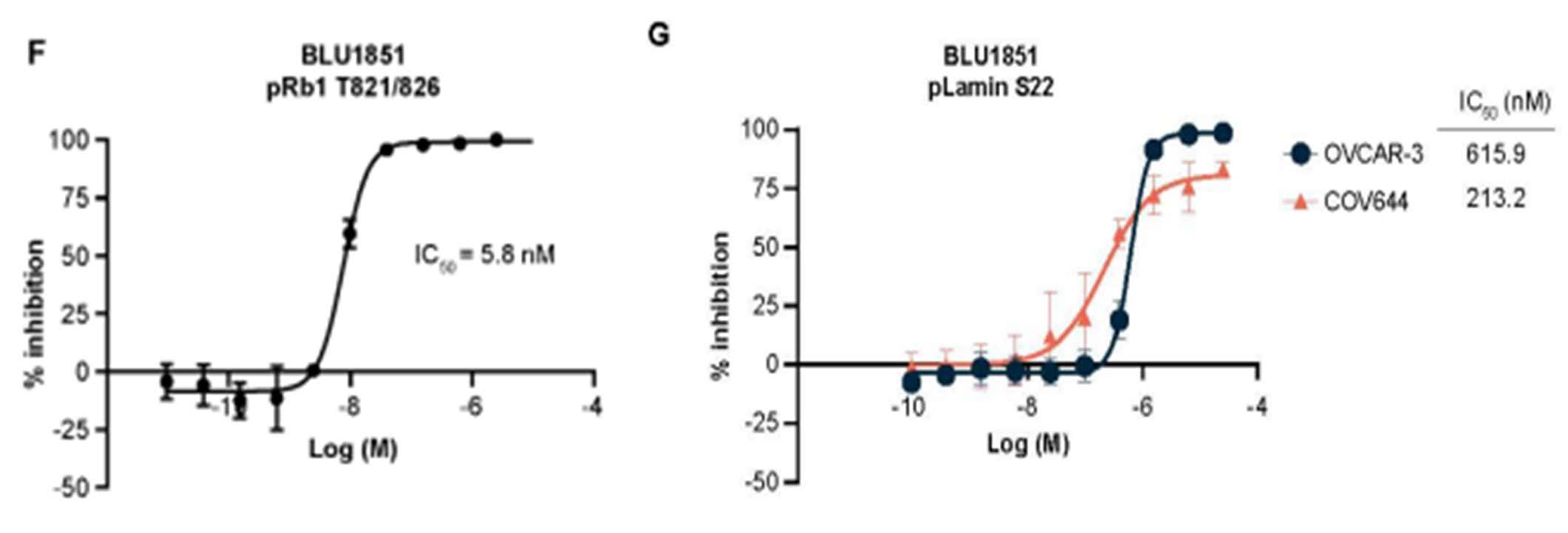
Figure 3. Using AlphaLISA Assays to measure phosphorylation status of key substrates involved in CHK1 signaling in OVCAR-3 and COV644 cells using a CDK2 inhibitor, BLU1851. To detect Retinoblastoma protein 1 (RB1) phosphorylation, 25,000 cells/well were plated overnight. For Lamin A/C phosphorylation, 3000 cells/well for OVCAR-3 or 500 cells/well for COV644 cells were incubated in substrate overnight. Note: Rb1 phosphorylation serves as a proxy for CDK2 activity, while lamin A/C phosphorylation indicates CDK1 activity.
Other Notable Findings by Brown et al.:
-
Synergism with DNA-Damaging Agents:
The researchers demonstrated that combining CDK2 inhibition with DNA-damaging agents synergistically killed CCNE1-amplified cancer cells. This suggests that CDK2 inhibitors could be used in combination with existing chemotherapy regimens to enhance their efficacy.
-
In Vivo Efficacy:
The preclinical study showed that CDK2 inhibition inhibited tumor growth in mouse xenograft models of CCNE1-amplified ovarian cancer. This in vivo efficacy supports the further clinical development of CDK2 inhibitors for this patient population.
Implications of the Study:
These findings position CDK2 as a critical driver of HR in CCNE1-amplified OC cells and as a promising therapeutic target. By selectively disrupting CDK2 activity with a selective inhibitor, the survival mechanisms of these cancer cells can be impaired, paving the way for novel and more effective treatments for patients with HGSOC.
About AlphaLISA Technology:
AlphaLISA (Amplified Luminescent Proximity Homogenous Assay) is a bead-based, no-wash homogeneous assay platform, enabling the detection of analytes and a dynamic range of biological interactions, including protein-protein, protein-peptide, and protein-small molecule interactions. This technology utilizes streptavidin-coated Alpha Donor beads and AlphaLISA Acceptor beads. Upon excitation, a singlet oxygen molecule is generated in the Donor bead that triggers a series of chemical reactions in the Acceptor bead, resulting in the emission of light at 615 nm, which is directly proportional to the amount of analyte or binding events within the sample. Alpha technology allows for the direct detection of molecules of interest in a homogeneous format, eliminating the need for time-consuming wash steps.
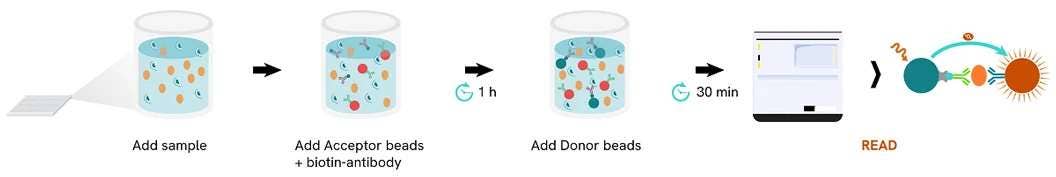
Figure 4. Workflow schematic demonstrating the ease of integrating AlphaLISA technology into your current research platform.
AlphaLISA® SureFire® Ultra™
The AlphaLISA SureFire Ultra kits are a subset of AlphaLISA technology offered by Revvity and are particularly suited for this type of cellular signaling research. These kits offer numerous advantages, including exceptional sensitivity, a wide dynamic range, and the ability to detect multiple targets using very few cells. These features make AlphaLISA an ideal platform for a broad array of applications, from basic research to drug discovery and development. For example, the AlphaLISA SureFire Ultra kits can be used to measure the phosphorylation of key substrates like CHK1, Rb, and lamin A/C, providing critical insights into the signaling pathways and efficacy of CDK2 inhibitors in CCNE1-amplified ovarian cancer cells.
-
AlphaLISA SureFire Ultra p-CDK2 (T160) Kit:
This kit measures the phosphorylation of CDK2 at threonine 160, a key activation site. It is crucial to understand how CDK2 activity is regulated in response to various signals and inhibitors.
-
AlphaLISA SureFire Ultra p-Rb (S807/S811) Kit:
The phosphorylation of retinoblastoma protein (Rb) at series 807 and 811 is an important downstream event of CDK2 activity. This kit can help quantify how CDK2 inhibition affects Rb phosphorylation and, consequently, cell cycle progression.
-
AlphaLISA SureFire Ultra Total CHK1 Kit:
While the phosphorylation-specific kit measures the active form of CHK1, this kit provides a measure of the total CHK1 protein levels, offering insights into the overall expression and stability of CHK1 in response to treatments.
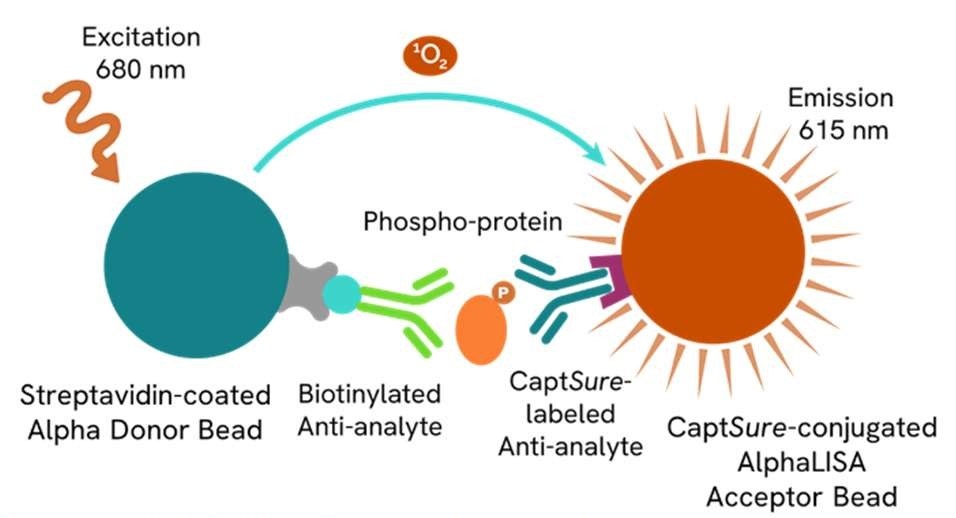
Figure 5. AlphaLISA® SureFire® Ultra™ Assay schematic demonstrating capture of a phosphorylated protein. The phosphorylated protein is detected in a sandwich assay using specific antibodies. One antibody is directed against a specific phospho-epitope on the analyte, while the other antibody is directed against another, non-phosphorylated, epitope on a distal part of the analyte. The resulting AlphaLISA signal in the immunoassay is directly proportional to the amount of phosphorylated analyte present in the sample.
By integrating AlphaLISA into research workflows, scientists can gain deeper insights into the molecular mechanisms underpinning CDK2 activity and its impact on cancer cell survival and proliferation.
Explore more here:
- AlphaLISA Technology Overview: Revvity - Alpha Assays
- AlphaLISA® SureFire® Ultra™ product list: Alpha SureFire Ultra no-wash immunoassay catalog | Revvity
References:
- Wang Y, Duval AJ, Adli M, Matei D. Biology-driven therapy advances in high-grade serous ovarian cancer. J Clin Invest. 2024 Jan 2;134(1). doi: 10.1172/JCI174013. PMID: 38165032; PMCID: PMC10760962.
- Brown VE, Moore SL, Chen M, House N, Ramsden P, Wu HJ, Ribich S, Grassian AR, Choi YJ. CDK2 regulates collapsed replication fork repair in CCNE1-amplified ovarian cancer cells via homologous recombination. NAR Cancer. 2023 Sep;5(3). doi: 10.1093/narcan/zcad039
- Chotiner JY, Wolgemuth DJ, Wang PJ. Functions of cyclins and CDKs in mammalian gametogenesis. Biol Reprod. 2019 Sep 1;101(3):591-601. doi: 10.1093/biolre/ioz070. PMID: 31078132; PMCID: PMC6791058.