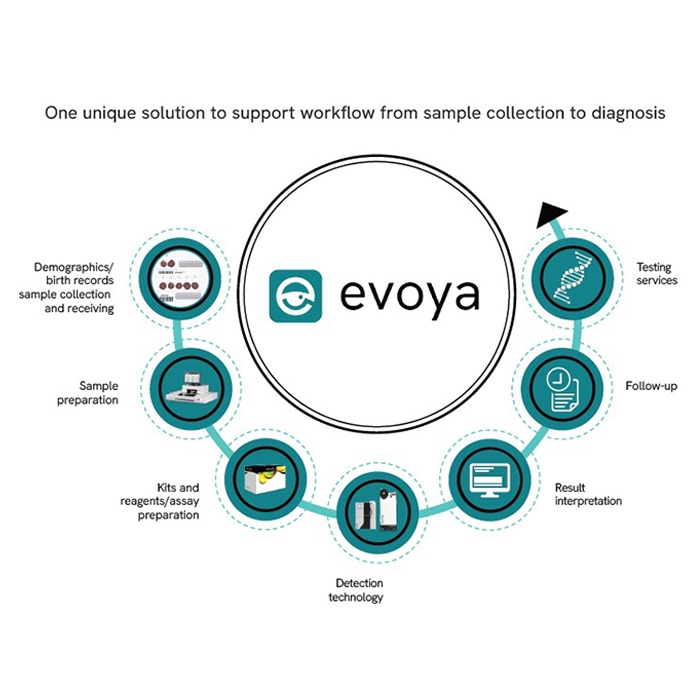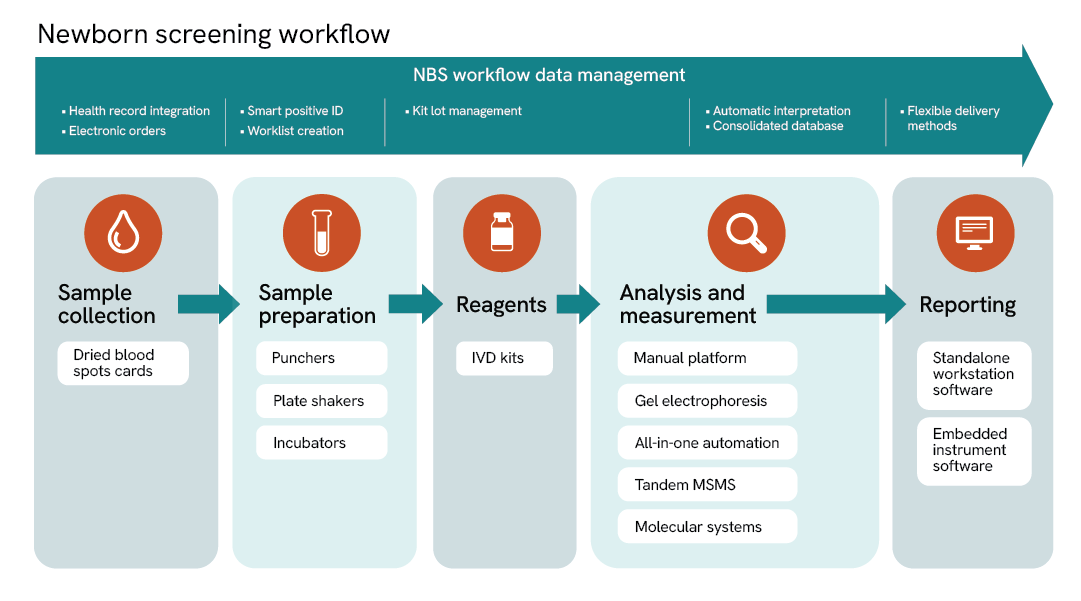

Complete integrated workflow
Sample collection
It all starts here.
An accurate sample is the first step in ensuring quality results later in a newborn screening workflow. Our streamlined dried blood spot sample (DBS) collection cards are designed to support accurate and reliable results.
The cards can be printed to the specific format of your lab, offering an easy and effective method for simple and precise DBS collection.
Sample preparation
Consistent practices in sample preparation contribute to reliable and reproducible test results,1 whether you are looking to simplify and automate your sample prep or use real time visualization to optimize your punching, our preparation instruments will support you in building stronger workflow screening processes.

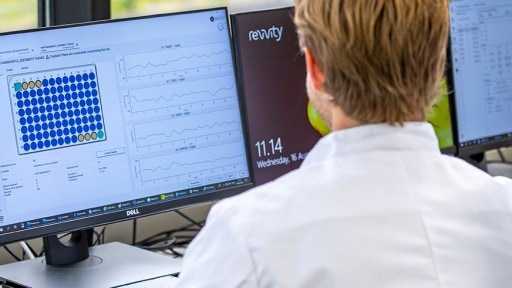
DELFIA Trio

DBS Puncher Instrument

Panthera Puncher 9 Instrument

Trinest Incubator shaker instrument
TriNEST incubator shaker is a high-quality solution for all well plate applications, where accurate and fast temperature and/or shaking control are needed.
Reference
- Ellison, S.L.R., Hardcastle, W.A. Causes of error in analytical chemistry: results of a web-based survey of proficiency testing participants. Accred Qual Assur 17, 453–464 (2012). doi.org/10.1007/s00769-012-0894-2
Analysis and measurement
Our range of newborn screening instruments offer scalable, customizable, integrated solutions that sit across MSMS, Molecular, Immunoassay and IEF.
From manual to fully automated workflows
Designed to be easy to use and deliver fast, accurate results with quality and performance standards built in, ensuring strong equipment uptime.

VICTOR2 D Instrument

Migele Gel Electrophoresis Unit

GSP Instrument (IVD)

Eonis Q96

QSight MD Screening Systems (IVD)
Reagents
We build high-quality, reliable reagents that support accurate detection of congenital disorders from the first days of life.
Our comprehensive reagent portfolio covers 50+ different disorders and integrates beautifully with our instruments across the newborn screening workflow.
Newborn Screening Kits
Use the highest quality reagents available today to screen for core newborn disorders as well as those detected by expanded screening.
Software
Nothing is more important than ensuring newborn screening data remains safe and secure.
As the unifying thread that runs throughout a newborn screening workflow, software systems like EVOYA and OZ Systems integrate fully to help you manage your data more flexibly in the cloud. These solutions provide quality checks and controls whilst ensuring patient results are flagged properly for follow-up.
All this whilst keeping NBS data secure through security and compliance standards.
Newborn Screening Software
Control and monitor the entire newborn screening process – including patient reporting and follow-up -- in one comprehensive information management system.
Helping babies survive and thrive
Newborn screening can prevent hundreds of thousands of instances of physical and mental disabilities and even deaths. As the global leader in newborn screening, over 800 million babies have been screened with our products over the last 28 years.
50+ conditions
are known to have good outcomes when detected and diagnosed early
85 newborns
get a healthier start to life everyday thanks to screening using our products
Explore our newborn screening products


Filters
1 - 25 of 73 Products and Services
DELFIA™ Trio is a 3-in-1 instrument developed for the sample processing of DELFIA manual kits.
Multiplexing of SMA, SCID and XLA screening can now be done in one assay utilizing Real-Time PCR technology, without increasing daily hands-on workload and complexity. This will provide a future-proof solution for cost-efficiently obtaining additional disorder information.
Early detection of metabolic and other inherited and rare disorders can improve the quality of life for at-risk babies and their families.
Revvity Transcribe AI, is an innovative OCR service designed to convert handwritten text on test request forms into a digitized format
Laboratory screening software that supports newborn screening workflows from sample collection to diagnosis and follow up offers numerous benefits for healthcare providers, researchers, and patients alike. Click here to watch video.
Neonatal Total Galactose kit is intended for the quantitative determination of total galactose (galactose and galactose-1-phosphate) concentrations in blood specimens dried on filter paper as an aid in screening newborns for galactosemia.
NEXTFLEX Neo NGS RUO kits offer robust genome coverage to identify variants in over 390 genes.
The RESOLVE™ Neonatal Hemoglobin test kit is designed to separate whole blood, cord blood or dried blood spot specimens for detection of normal and variant hemoglobins by isoelectric focusing.
DELFIA Neonatal 17α-OH-progesterone kit.
Neonatal Galactose Transferase (GALT) kits are intended for the semi-quantitative determination of galactose-1-phosphate uridyltransferase (GALT) activity in blood specimens collected onto filter paper, in screening newborns for galactosemia.
Revvity 226 Research Sample Collection Devices include the high quality Ahlstrom 226 grade paper ensuring even and uniform sample distribution.
Migele™ Gel Electrophoresis Unit works with the RESOLVE™ hemoglobin kit to detect hemoglobinopathies in newborns and adults.
The DBS Puncher has been designed to automatically punch dry blood spot samples into microtitration plates for processing.
The GSP® is a high throughput batch analyzer intended for quantitative or qualitative measurement of neonatal screening samples.
Panthera Puncher™ 9 is the new generation dried sample punching device for automatically punching dry blood spot samples into microtitration plates.
The EONIS™ Q 384-well variant instrument has been designed to have a simple and unique workflow, making training an implementation of qPCR easy to implement. Due to the simplified workflow the EONIS Q boasts a less than 2-hour turnaround time, with no need for a clean room.
TriNEST™ incubator shaker is a high-quality solution for all well plate applications, where accurate and fast temperature and/or shaking control are needed. TriNEST is a first-class choice for e.g. primer extension and DNA hybridization. For rapid cooling, a cooling block is also available.
The VICTOR2™ D fluorometer is designed for all Revvity diagnostic and screening assays based on either time-resolved fluorescence or prompt fluorescence.
Migele™ Gel Electrophoresis Unit works with the RESOLVE™ hemoglobin kit to detect hemoglobinopathies in newborns and adults.
The Eonis™ Q 96-well variant instrument has been designed to provide a simple and unique workflow, making training and implementation of qPCR easy to deliver. Due to the simplified workflow the Eonis Q boasts a less than 3-hour turnaround time, with no need for a clean room.
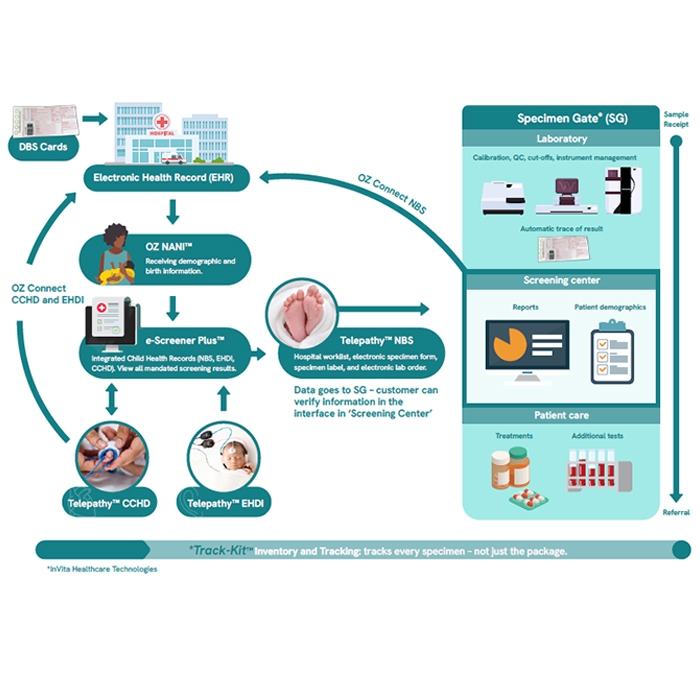
A hearing health information system from newborn screening through to diagnosis and intervention. Used in state public health programs, large hospital systems or integrated delivery networks to manage all aspects of an Early Hearing Detection and Intervention (EHDI) program including communications, letters, consent, care coordination and reporting. The eSP™ EHDI information management system captures direct results from screening devices, minimizing staff manual data entry. Includes ability to notify Early Intervention if a patient has been assigned to them.
OZ eSP™ is a hosted, cloud-based database and the foundational infrastructure required for all OZ products. All newborn screening data are stored in and exchanged via the OZ eSP database. Each newborn has a screening record with integrated results. Includes Deduplication reporting.
Within OZ eSP™ this module facilitates cohesive monitoring and reporting of birth conditions/birth defects. It collects newborn program data on birth defects and reportable birth conditions. These data are stored in the eSP integrated child health record.
Integrating the Healthcare Enterprise (IHE) NANI Technical Profile is based on HL7 ADT (Admission, Discharge and Transfer) electronic messages. This messaging system enables a newborn’s demographics information from the hospital EHR system to OZ solutions with necessary information for newborn screening. The hospital or healthcare system provides VPN connectivity to OZ Systems.
This cloud-based software provides package manifest and label as well as tracks each specimen once it leaves the hospital and is delivered to the state lab by the courier. It integrates with Telepathy NBS to provide expiration alerts for specimen cards, the status of the specimen (where it is in the process of delivery) as well as alerts for specimens (late delivery). OZ Track-Kit™ also provides inventory management as well as specimen storage and destruction information.
Filters
1 - 25 of 38 Resources
Measurement of alloisoleucine and branched-chain amino acids in dried blood spot using QSight
Measurement of isobaric-c5 acylcarnitines in dried blood spots using QSight 225 MD UHPLC
Measurement of methylmalonic acid and 3-hydroxypropionic acid in dried blood using QSight
Optimization and miniaturization of DELFIA TRF assays converted from ELISA.
Questions?
We’re here to help.
Contact us Sign up to the newsletter
Click hereRevvity does not endorse or make recommendations with respect to research, medication, or treatments. All information presented is for informational purposes only and is not intended as medical advice. For country specific recommendations please consult your local health care professionals.
Products may not be licensed in accordance with the laws in all countries, such as the United States and Canada. Please check with your local representative for availability. Please note that product labeling (such as kit insert, product label, and kit box) may be different compared to the company branding. Please contact your local representative for further details.