

HostDetect E.coli PCR DNA Quant Kit

HostDetect E.coli PCR DNA Quant Kit




This real-time PCR assay (RUO) enables quantitative detection of residual E.coli host genomic DNA in extracted nucleic acid samples from biopharmaceutical products.
| Feature | Specification |
|---|---|
| Analytical Sensitivity | > 0.03 pg/rxn |
This real-time PCR assay (RUO) enables quantitative detection of residual E.coli host genomic DNA in extracted nucleic acid samples from biopharmaceutical products.


HostDetect E.coli PCR DNA Quant Kit


HostDetect E.coli PCR DNA Quant Kit


Product information
Overview
The HostDetect™ E.coli PCR DNA Quant Kit is specific for DNA from E.coli genome. The reagents utilize sequence-specific primers and TaqMan® probe to amplify the 16S ribosomal RNA gene of E.coli genomic DNA for residual host genomic identification. A primer/probe set to detect Internal Control (IC), is also included for monitoring entire process from extraction to real-time PCR. The reagents also use a dUTP/UNG carryover prevention system to avoid contamination of PCR products. For more information, contact: BioprocessQC@revvity.com
Key Highlights:
- Quantify E.coli genomic DNA with as little as 0.03 pg per PCR reaction in < 2 hours of PCR
- Internal control detection for process monitoring from sample extraction to PCR
- High specificity: No cross-reactivity with unrelated DNA
- Flexible extracted DNA input volume up to 10 µL per well
- Scalable reaction numbers from 1 to up to 192 per kit
Additional product information
HostDetect™ E.coli PCR DNA Quant Kit Workflow

The probes for the E.coli genomic DNA and Internal Control detection are labeled with FAM and HEX/VIC fluorescent dyes, respectively, to generate target-specific signal. ROX is used for Passive Reference. Any Real-time qPCR Instruments with FAM™ and VIC®/HEX™ channels can be used with this assay.
HostDetect™ E.coli PCR DNA Quant Kit Quantification Range**
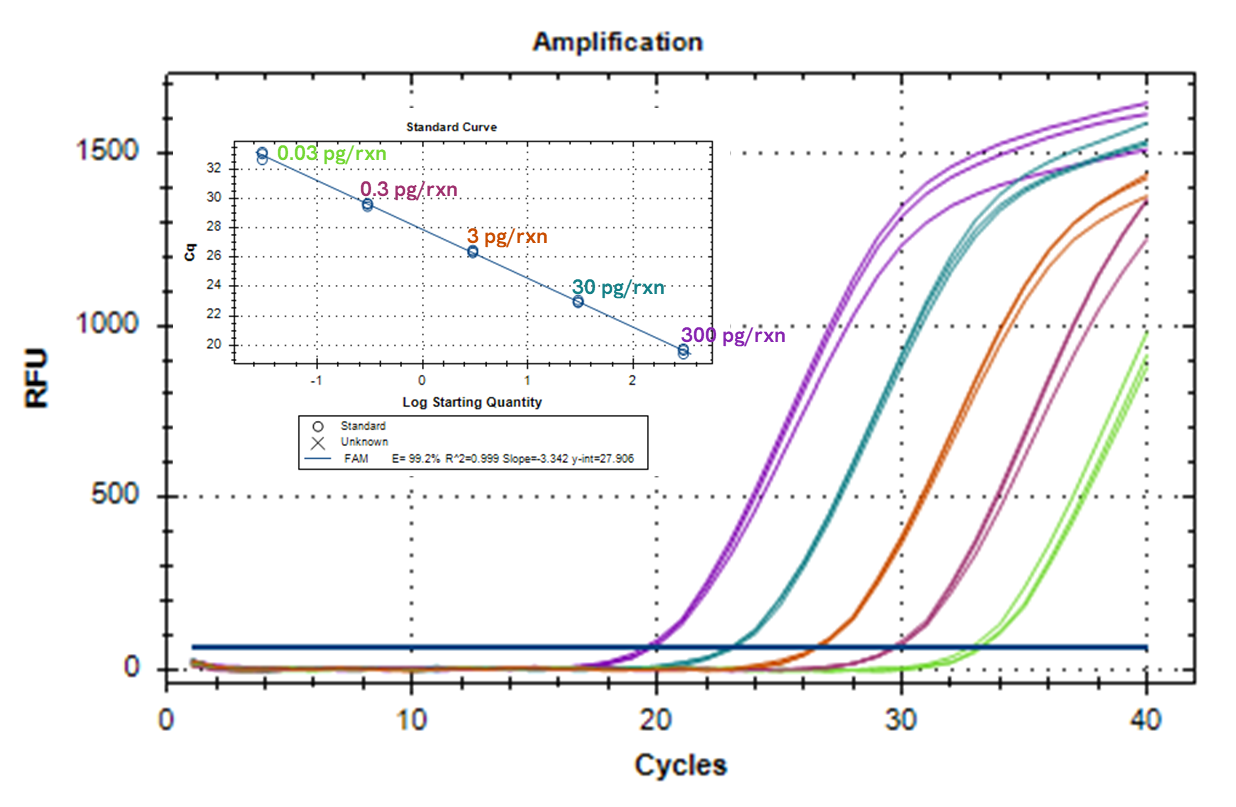
**: ATCC or USP reference standards for residual DNAs are not included in the kit. Customer must order these separately from ATCC or USP web sites.
Features:
| Quantification Range | 0.3 ng to 0.03 pg/rxn |
|---|---|
| Linearity | R2 ≥ 0.99, Eff%= 100±20% |
| Sensitivity | ≥ 0.03 pg/rxn |
| Precision | CV < 5% at 0.03 pg/rxn |
| Shipping Conditions | Shipped on Dry ice |
| Storage Conditions | -25 to -15 °C |
Specifications
| Analytical Sensitivity |
> 0.03 pg/rxn
|
|---|---|
| Shipping Conditions |
Shipped in Dry Ice
|
| Storage Conditions |
-25C to -15C
|
| Unit Size |
192 rxns
|
Resources
Are you looking for resources, click on the resource type to explore further.
"Residual HEK293 DNA is a critical impurity that must be minimized when generating recombinant AAV (rAAV) therapeutic products, as...
SDS, COAs, Manuals and more
Are you looking for technical documents related to the product? We have categorized them in dedicated sections below. Explore now.
- LanguageEnglishCountryUnited States
- LanguageEnglishCountryEU
- Resource TypeInstructions for Use - IFULanguageEnglishCountry-


How can we help you?
We are here to answer your questions.








































