
AlphaLISA Human CCL17 Detection Kit, 100 Assay Points
AlphaLISA Human CCL17 Detection Kit, 100 Assay Points



The AlphaLISA™ Human CCL17 kit is designed for the simple and rapid quantification of soluble CCL17 (TARC) in cell supernatants, providing a fast no-wash alternative to traditional wash-based ELISA assays.
| Feature | Specification |
|---|---|
| Protocol Time | 1.5h at RT |
| Sample Volume | 10 µL |
The AlphaLISA™ Human CCL17 kit is designed for the simple and rapid quantification of soluble CCL17 (TARC) in cell supernatants, providing a fast no-wash alternative to traditional wash-based ELISA assays.


AlphaLISA Human CCL17 Detection Kit, 100 Assay Points


AlphaLISA Human CCL17 Detection Kit, 100 Assay Points


Product information
Overview
Human CCL17, also known as thymus and activation regulated chemokine (TARC), was initially isolated from phytohemagglutinin-stimulated peripheral blood mononuclear cells. CCL17 is constitutively expressed in the thymus and under activation in several cell types. CCL17-mediated recruitment of Th2 cells and CLA+ CD4+ T cells, plays a key role in allergic diseases such as atopic dermatitis, allergic asthma, allergic rhinitis, and allergic contact dermatitis. In addition, CCL17 has been detected in idiopathic pulmonary fibrosis. CCL17 and CCL22 secreted by dendritic cells (DCs) seems to mediate the recruitment of regulatory T cells to sites of inflammation in patients with chronic hepatitis. Increased levels of serum CCL17 is associated with a greater risk of developing atherosclerosis.
Formats
- Our 100 assay point kit allows you to run 100 wells in 96-well format, using a 100 µL reaction volume (10 µL of sample).
- Our 500 assay point kit allows you to run 500 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
- Our 5,000 assay point kit allows you to run 5,000 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
Features
- No-wash steps, no separation steps
- ELISA alternative technology
- Sensitive detection
- Broad sample compatibility
- Small sample volume
- Results in less than 3 hours
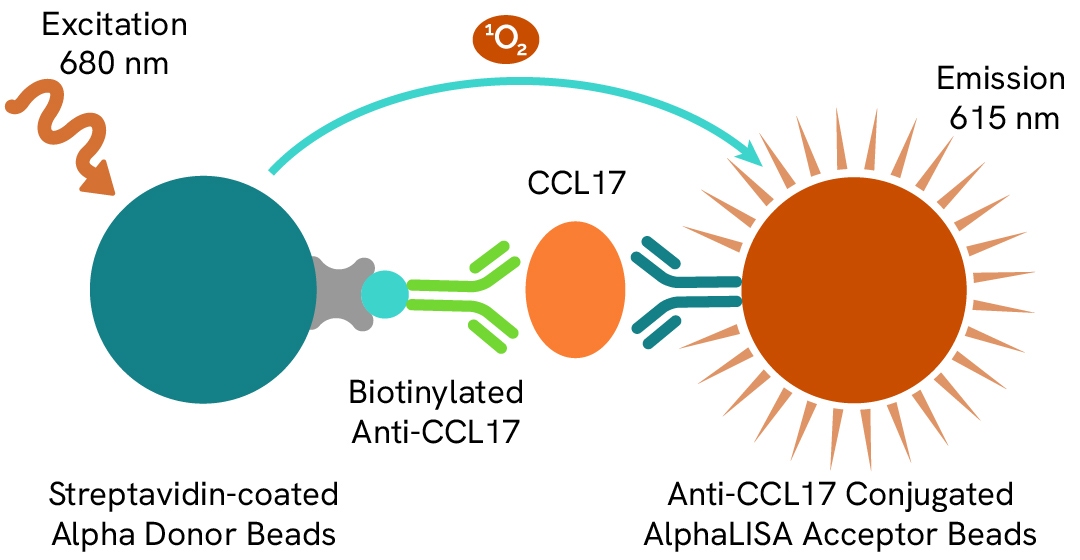
AlphaLISA technology allows the detection of molecules of interest in a no-wash, highly sensitive, quantitative assay. In an AlphaLISA assay, a biotinylated anti-analyte antibody binds to the Streptavidin-coated Donor beads while another anti-analyte antibody is conjugated to AlphaLISA Acceptor beads. In the presence of the analyte, the beads come into close proximity. The excitation of the Donor beads causes the release of singlet oxygen molecules that triggers a cascade of energy transfer in the Acceptor beads, resulting in a sharp peak of light emission at 615 nm.
Specifications
| Automation Compatible |
Yes
|
|---|---|
| Brand |
AlphaLISA
|
| Detection Modality |
Alpha
|
| Protocol Time |
1.5h at RT
|
| Sample Volume |
10 µL
|
| Shipping Conditions |
Shipped in Blue Ice
|
| Target |
CCL17
|
| Target Class |
Cytokines
|
| Target Species |
Human
|
| Technology |
Alpha
|
| Therapeutic Area |
Inflammation
|
| Unit Size |
100 assay points
|
How it works
Principle of the AlphaLISA human CCL17 assay
The AlphaLISA human CCL17 assay is based on an AlphaLISA sandwich immunoassay involving a biotinylated anti-target antibody bound to Streptavidin-coated AlphaLISA Donor beads and an anti-target antibody conjugated to AlphaLISA Acceptor beads. Both antibodies are directed against the CCL17 protein. In the presence of the target, both antibodies bind to CCL17 and the beads come into proximity. The excitation of the Donor beads provokes the release of singlet oxygen molecules that triggers a cascade of energy transfer within the Acceptor beads, resulting in emission with λmax at 615 nm. The intensity of the signal is directly proportional to the concentration of CCL17 present in the sample.

Protocol of the AlphaLISA human CCL17 assay
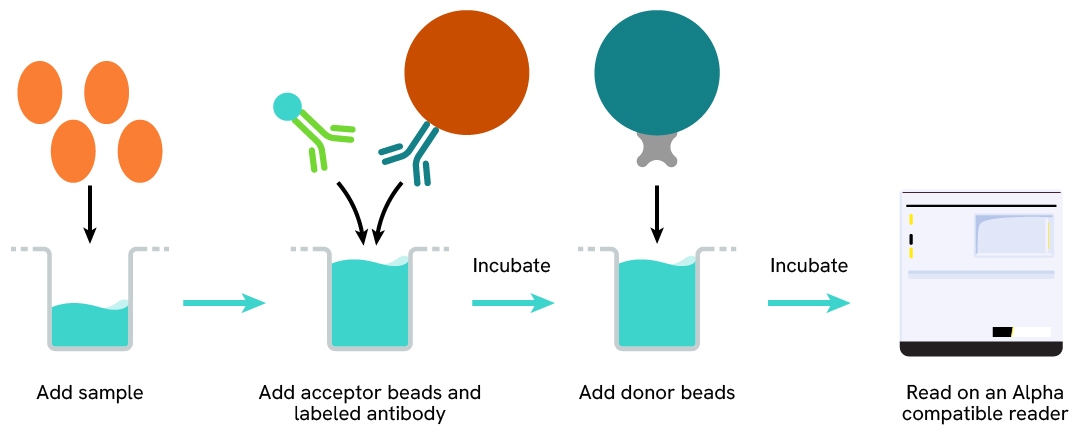
The AlphaLISA human CCL17 assay can be run in a 96- or 384-well detection plate (50 µL final). As described here, samples (cell supernatants or fluids) or standards are dispensed directly into the assay plate for the detection of CCL17 by AlphaLISA reagents. No washing steps are needed. The protocol can be further miniaturized or upscaled by simply resizing each addition volume proportionally.
Assay details
Human CCL17 assay details
| Time to result | 1.5 hours at RT |
|---|---|
| Kit component | Lyophilized analyte, SA-Donor Beads, Biotinylated anti-analyte, Anti-analyte conjugated Acceptor Beads, Assay Buffer |
| Species | Human only |
| LDL | 0.3 pg/mL |
| LLOQ | 0.9 pg/mL |
| Dynamic range | 1-10,000 pg/mL |
| Calibration | No international standard available |
Analytical performance
Intra-assay precision table
Each of the 3 samples was measured 24 times, and the % CV was calculated for each sample. Samples were supernatants from PHA-treated PBMCs.
| Sample | [CCL17] (pg/mL) | CV |
|---|---|---|
| 1 | 1389 | 10.1% |
| 2 | 742 | 9.0% |
| 3 | 305 | 6.7% |
| Mean CV | 8.6% | |
Inter-assay precision table
Each of the samples was measured in 3 independent experiments (3 days), and the % CV was calculated for each sample. Samples were supernatants from PHA-treated PBMCs.
| Sample | [CCL17] (pg/mL) | CV |
|---|---|---|
| 1 | 1436 | 10.5% |
| 2 | 696 | 14.6% |
| 3 | 338 | 11.4% |
| Mean CV | 12.1% | |
Spike and recovery table
Each of the samples was measured in 3 independent experiments (3 days), and the % CV was calculated for each sample. Samples were supernatants from PHA-treated PBMCs spiked with CCL17 recombinant protein.
| Spiked CCL17 (pg/mL) | % Recovery | ||
|---|---|---|---|
| DMEM+10% FBS | RPMI+10% FBS | 100% FBS | |
| 375 | 106% | 99% | 109% |
| 250 | 101% | 94% | 111% |
| 125 | 103% | 91% | 112% |
Dilutional linearity table
Each of the samples was measured in 3 independent experiments (3 days), and the % CV was calculated for each sample. Samples were supernatants from PHA-treated PBMCs.
| Sample dilution factor (x) | Expected CCL17] (pg/mL) | Observed CCL17] (pg/mL) | Dilution recovery (%) |
|---|---|---|---|
| neat | 1431 | 1217 | 90% |
| 1/2 | 562 | 608 | 109% |
| 1/4 | 300 | 304 | 101% |
| 1/8 | 148 | 152 | 102% |
| 1/16 | 77 | 76 | 98% |
| 1/32 | 37 | 38 | 103% |
Selectivity table
Cross reactivities were assessed using recombinant CCL17 proteins from different species. Standard curves were generated for each protein diluted in the kit diluent for recombinant proteins.
Cross-reactivity percentage was computed by comparing the Alpha signal at the highest concentration (30,000 pg/mL) to the human protein. The assay is specific for human CCL17.
| Proteins | Cross reactivity (%) |
|---|---|
| mouse | 0.00 |
| rat | 0.00 |
Assay validation
Cell densities of PHA stimulated PBMCs
Human PBMCs were stimulated with 10 µg/mL of PHA in RPMI for 24h, in a humidified atmosphere at 37°C and 5% CO2. Different cell densities were tested: 250, 500 and 750 kcells/well were plated in a 96-well plate.
After 24h stimulation, the cell supernatants were collected and 5 µL of each sample was transferred into an AlphaPlate-384 to measure the secreted CCL17 in each sample. The standard curve was prepared in RPMI. All other assay components diluted in AlphaLISA Immunoassay Buffer were added into the wells following the kit protocol. After the final 30 minutes of incubation with Alpha Streptavidin Donor beads, the AlphaLISA counts were measured and the CCL17 concentration in each supernatant sample was interpolated from the standard curve.
As expected, the secretion of CCL17 increases with PBMCs cell density.
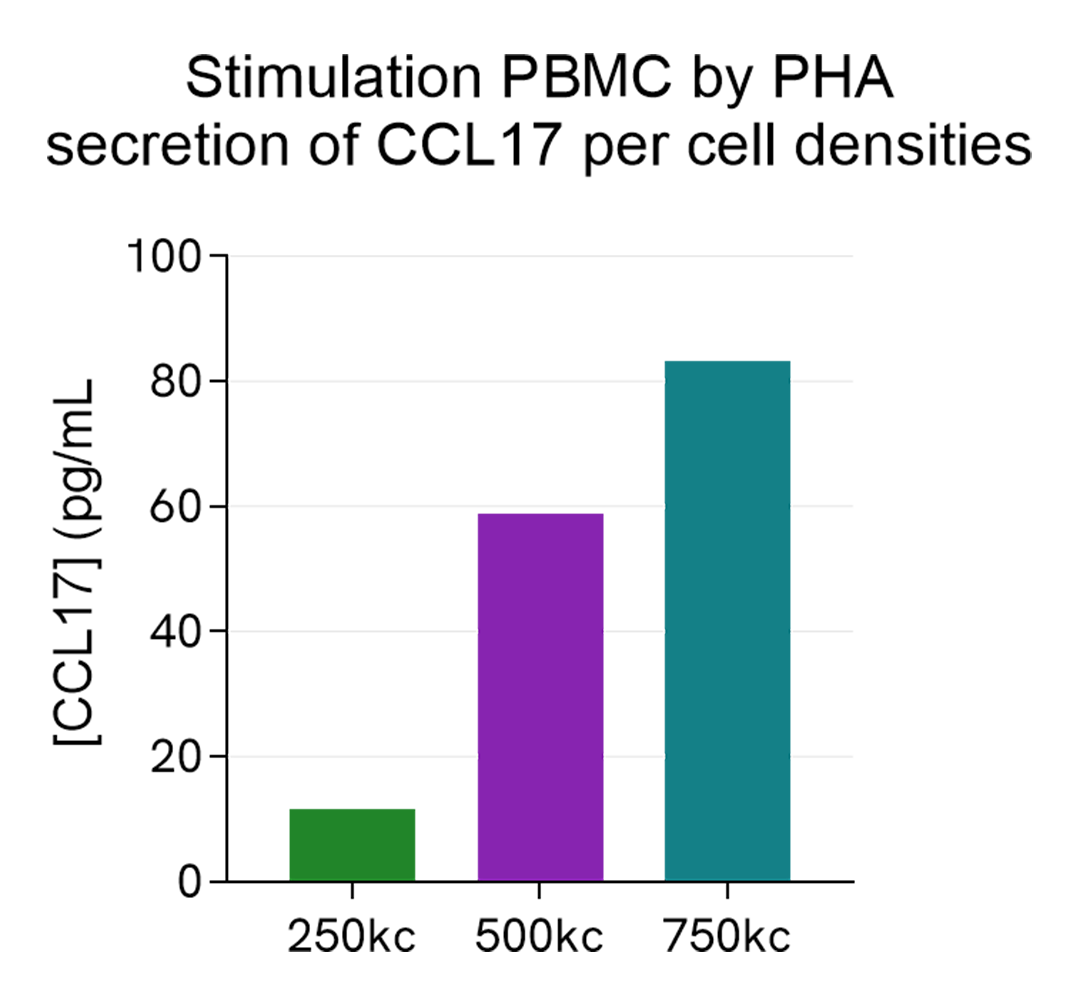
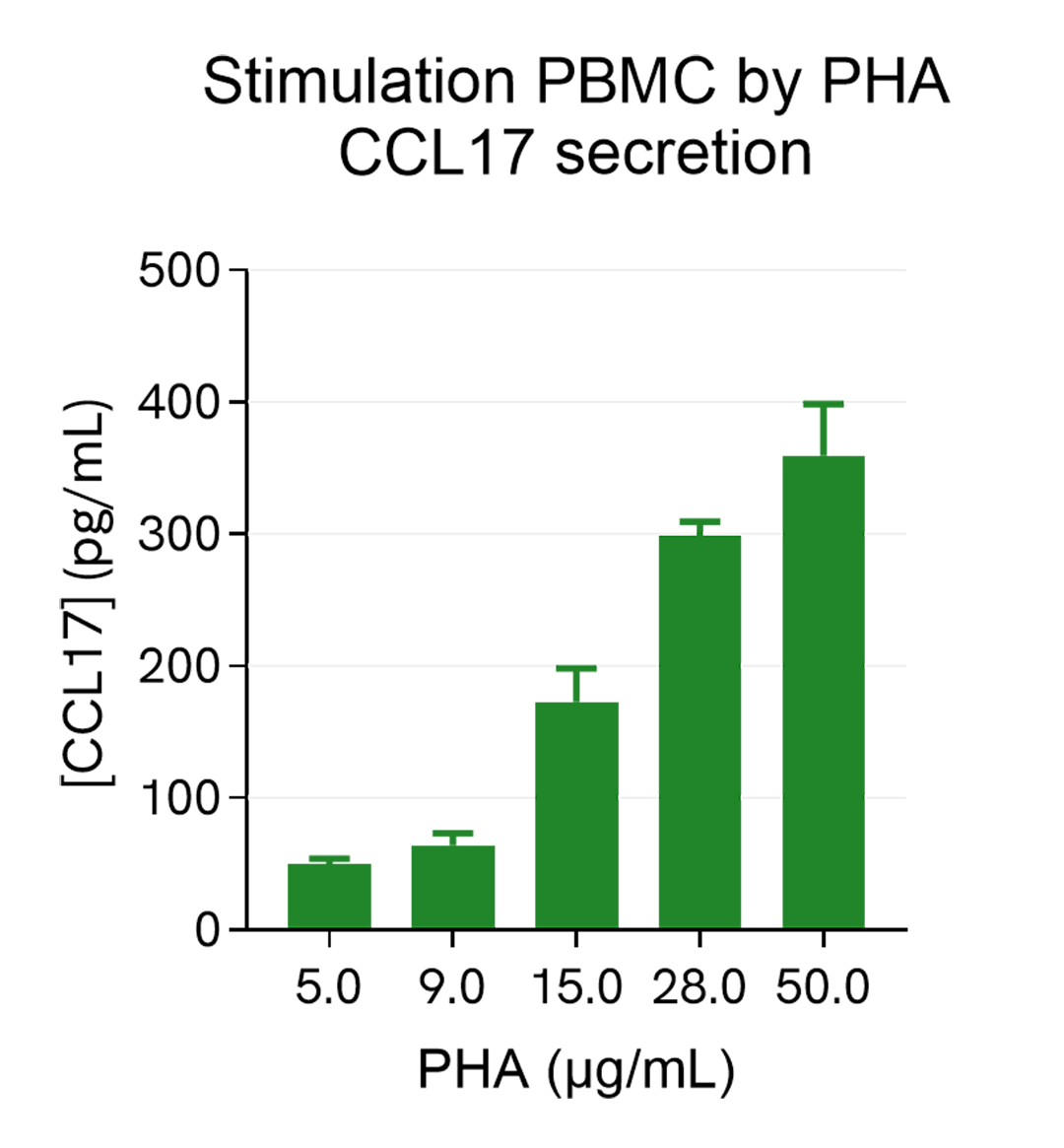
Dose response of PHA on PBMC
Human PBMCs (750,000 cells/well in RPMI) were stimulated with increasing concentrations of PHA for 24h, in a humidified atmospher3 at 37°C and 5% CO2. After 24h stimulation, the cell supernatants were collected and 5 µL of each sample was transferred into an AlphaPlate-384 to measure the secreted CCL17 in each sample. The standard curve was prepared in RPMI. All other assay components diluted in AlphaLISA Immunoassay Buffer were added into the wells using the kit protocol. After the final 30 minutes of incubation with Alpha Streptavidin Donor beads the AlphaLISA counts were measured and the CCL17 concentration in each supernatant sample was interpolated from the standard curve. As expected, the CCL17 secretion increases with PHA concentrations and reaches a plateau (around 30µg/mL).
Synergistic effect of co-stimulation of IL4 and LPS
Human PBMCs (750,000 cells/well in RPMI) were stimulated with 20 ng/mL of IL4 or 2 µg/mL of LPS or co-stimulated with both IL4 and LPS; The plate was placed in a humidified atmosphere at 37°C and 5% CO2.
After 24h stimulation, the cell supernatants were collected and 5 µL of each sample was transferred into an AlphaPlate-384 to measure the secreted CCL17 in each sample. The standard curve was prepared in RPMI. All other assay components diluted in AlphaLISA Immunoassay Buffer were added into the wells using the kit protocol. After the final 30 minutes of incubation with Alpha Streptavidin Donor beads, the AlphaLISA counts were measured and the CCL17 concentration in each supernatant sample was extrapolated from the standard curve. IL4 stimulates the secretion of CCL17 in PBMCs while LPS alone does not. However, there is a synergy effect of the co-stimulation of LPS and IL4, resulting in more secretion of CCL17 than IL4 alone, in agreement with the literature (J. Immunol. (2017) 198 (9): 3426-3435 ).
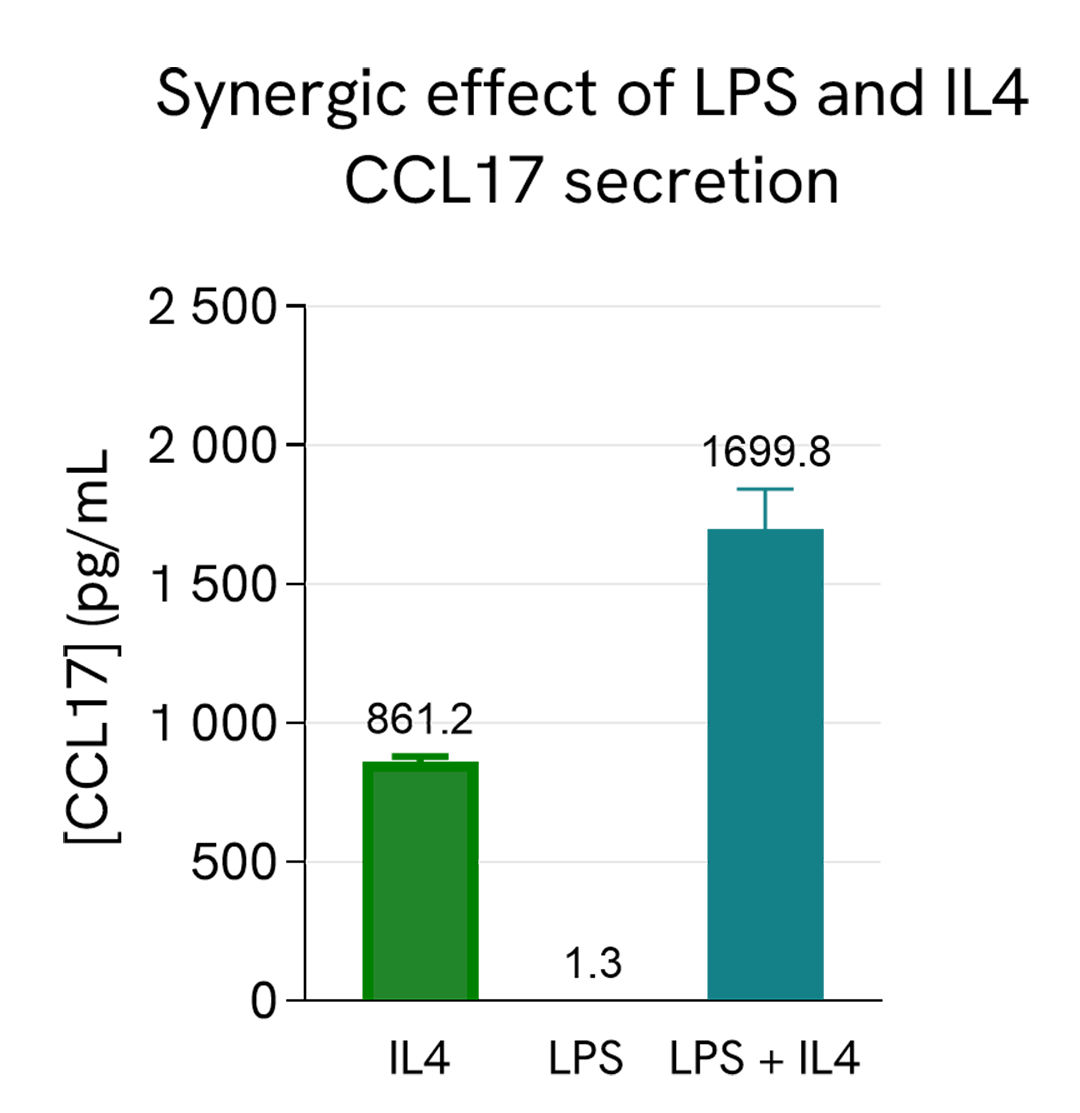
Loading...


How can we help you?
We are here to answer your questions.







































