The T-SPOT™.CMV test
The T-SPOT.CMV test is an in vitro diagnostic test intended to be used to assess a patient’s level of anti-CMV cell-mediated immunity. The test is a simplified variant of the ELISPOT assay technique.
ELISPOT assays are exceptionally sensitive since the target cytokine is captured directly around the secreting cell, before it is diluted in the supernatant, bound by receptors of adjacent cells or degraded. This makes ELISPOT assays much more sensitive than conventional ELISA assays.
The T-SPOT.CMV test is designed for the detection of effector T cells that respond to stimulation by antigens specific for cytomegalovirus (CMV) by releasing cytokines. The test enumerates individual activated T cells and is suitable for use with patients regardless of age, sex, ethnicity, therapy or immune status.
Featured resources
The T-SPOT technology
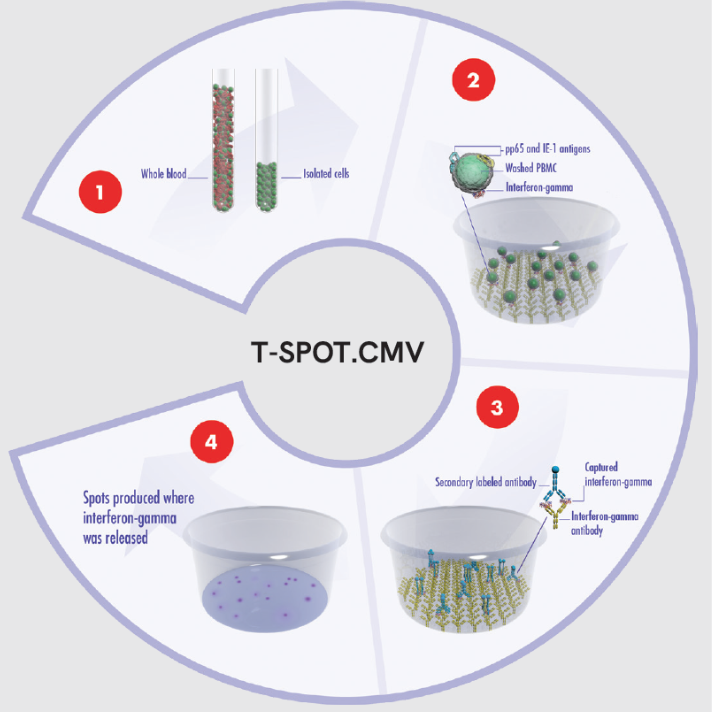
The T-SPOT.CMV test utilizes T-SPOT technology to isolate and stimulate the immune cells with CMV specific antigens. It measures the immune cell responses at a single cell level and detects T cell activation through interferon-gamma release to assess the patient’s immune system.
- A blood sample is collected using routine phlebotomy and a standard blood collection tube from which a subset of white blood cells, known as peripheral blood mononuclear cells (PBMC), are isolated. The cells are washed remove any sources of background interfering signal. The PBMCs are then counted and normalized to create a standard cell suspension so that a standardised cell number is used in the test. This ensures that even with low T cell titres due to weakened immune systems (the immunocompromised and immunosuppressed) there are adequate numbers of PBMCs added to the microtiter wells.
- A standard number of cells are added into specially designed plates and stimulated with CMV-specific antigens pp65 and IE-1. Cells responding to these antigens release the cytokine interferon-gamma.
- Interferon-gamma antibodies are used to directly capture interferon-gamma as it is released by the cells. A secondary labelled antibody is added and binds to the captured interferon-gamma.
- A detection reagent is added and reacts with the secondary labelled antibody. This reaction produces spots, which are a footprint of where the interferon-gamma was released. Spots are then enumerated.
Featured products
Products may not be licensed in accordance with the laws in all countries. Please check with your local representative for availability.
References:
- National Institute of Allergy and Infectious Diseases. Overview of the Immune System.
- Abbas AK, et al. Cellular and Molecular Immunology. 9th edition. Elsevier; 2017
- Egli A, et al. State-of-the-Art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clin Infect Dis. 2012;55(12):1678-1689
- Espigado I, Vicente F, et al. Timing of CMV-specific effector memory T cells predicts viral replication and survival after allogeneic hematopoietic stem cell transplantation. Transplant International. 2014;27(12):1253-1262.
- Abate D, Saldan A, Fiscon M, et al. Evaluation of cytomegalovirus (CMV)-specific T cell immune reconstitution revealed that baseline antiviral immunity, prophylaxis, or preemptive therapy but not antithymocyte globulin treatment contribute to CMV-specific T cell reconstitution in kidney transplant recipients. J Infect Dis. 2010;202(4):585-594.