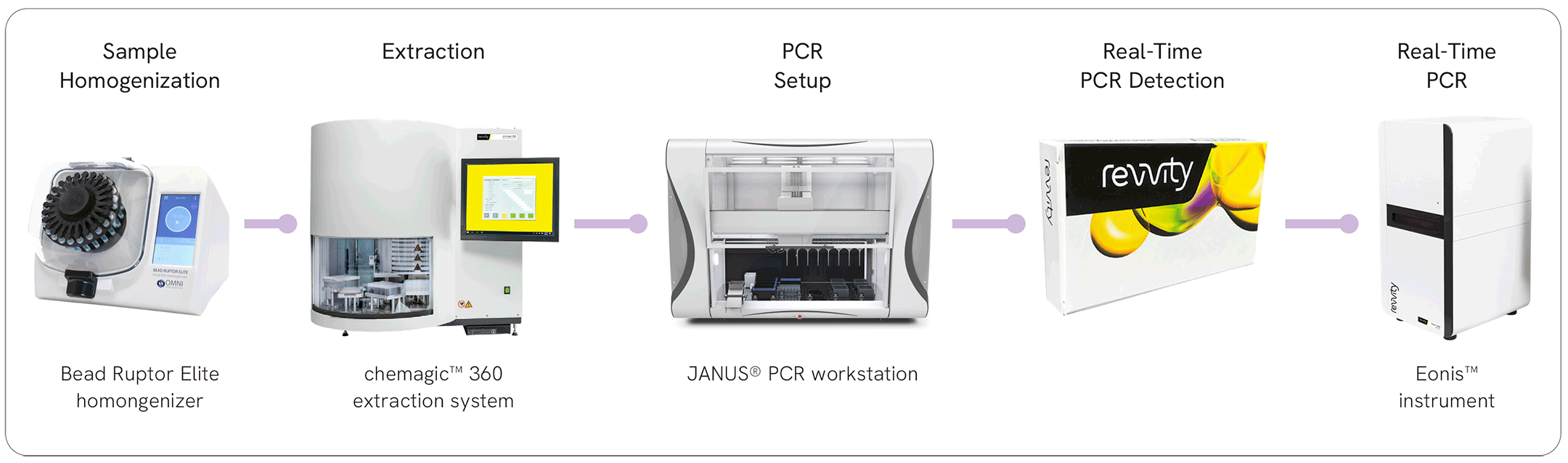
Sample homogenization
Jumpstart your qPCR analysis with a robust and powerful homogenizer designed to simplify sample preparation and prepare high quality lysates prior to nucleic acid extraction.
Nucleic acid isolation for qPCR analysis
The isolation of DNA and RNA from diverse sample materials is a crucial part of the qPCR workflow. Learn more about Revvity’s solution for high performance nucleic acid isolation.
qPCR set-up
The automation of PCR setup increases throughput and reproducibility while greatly reducing hands-on time. Our portfolio of liquid handlers enables the rapid and precise setup of your qPCR experiments.
Mpox virus real-time PCR assay
The Mpox Virus Real-Time PCR Kit is a real-time PCR test (RUO) intended for the qualitative detection of mpox virus (formerly known as monkeypox virus, MPXV) from extracted nucleic acid.
Candida auris real-time PCR reagents
The Candida auris detection real-time PCR reagents is a real-time PCR assay (RUO) for qualitative detection of Candida auris (C. auris) from extracted nucleic acid samples collected from human skin swabs, environmental surface swabs, or laboratory cultures.
cCMV real-time PCR assay
The research use only NeoMDx™ cCMV real-time PCR assay is a multiplexed, real-time qPCR test delivering qualitative detection of congenital cytomegalovirus (cCMV) and the RPP30 (Ribonuclease P/MRP Subunit P30, RNase P) gene in DNA from dried blood spots (DBS).


Filters
1 - 12 of 12 Products and Services
The EONIS™ Q 384-well variant instrument has been designed to have a simple and unique workflow, making training an implementation of qPCR easy to implement. Due to the simplified workflow the EONIS Q boasts a less than 2-hour turnaround time, with no need for a clean room.
This real-time PCR assay (RUO) enables quantitative detection of residual E.coli host genomic DNA in extracted nucleic acid samples from biopharmaceutical products.
This real-time PCR assay (RUO) enables quantitative detection of residual HEK293 host genomic DNA in extracted nucleic acid samples from biopharmaceutical products.
This real-time PCR assay (RUO) enables quantitative detection of residual CHO host genomic DNA in extracted nucleic acid samples from biopharmaceutical products.
Positive controls to be used with Revvity's Mpox Virus Real-Time PCR kit.
The Mpox Virus Real-Time PCR kit is intended for the qualitative detection of mpox virus DNA (Clade I/II).
cCMV dry chemistry real-time PCR assay kit for the Eonis™ System. This is a research for use only kit
NeoMDx™ cCMV PCR Reagent Kit with 96 tests per kit
This real-time PCR assay (RUO) enables qualitative detection of Candida auris (C. auris) from extracted nucleic acid samples collected from human skin swabs, environmental surface swabs, or laboratory cultures.