Residual DNA detection assays
Our bioprocess quality control solutions are designed to streamline your host cell residual DNA detection workflow, from sample extraction to qPCR quantification. Helping you achieve the purity of your biotherapeutic and CGT products with assays that can demonstrate whether contaminant levels are in compliance with critical quality attributes.

HostDetect CHO PCR DNA Quant Kit

HostDetect HEK293 PCR DNA Quant Kit

HostDetect E.coli PCR DNA Quant Kit
Host cell protein detection assays
During biotherapeutic manufacturing and production, CHO, HEK 293 and E.coli host cells produce HCP impurities which, if not removed, can induce immunogenicity in individuals or reduce the potency, stability, or effectiveness of a drug. Therefore, to meet regulatory organizations’ guidelines we have developed a range of HCP detection assays to improve your workflows.

HTRF CHO HCP Detection Kit, 500 Assay Points
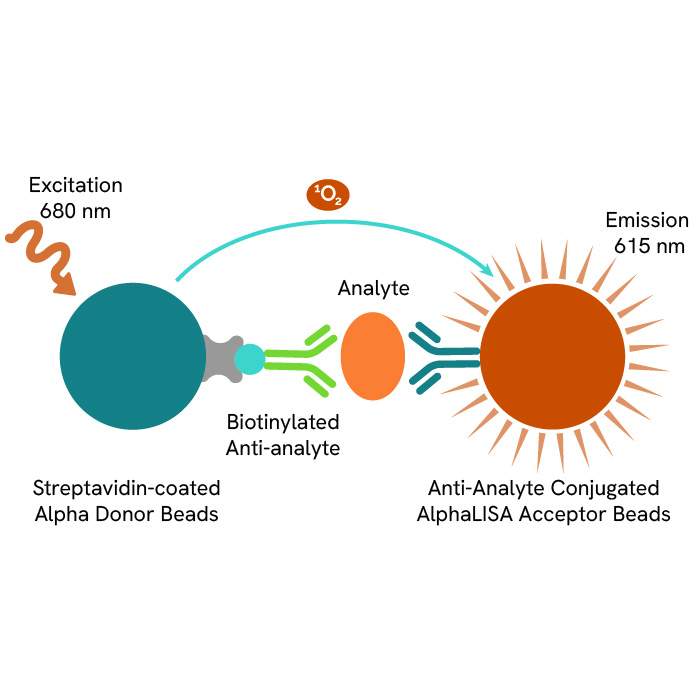
AlphaLISA HEK 293 HCP Detection Kit, 100 Assay Points

AlphaLISA CHO HCP Detection Kit, 100 Assay Points
Protein contamination detection technology
To aid rapid protein contamination detection, utilize an automated microfluidic analysis platform that can support FDA 21 CFR Part 11 regulations. By providing quantitative results for contaminants while using minimal sample volumes, the LabChip™ protein characterization system is invaluable in the biopharmaceutical manufacturing workflow by significantly enhancing protein purity assessment and helping you realize higher quality standards.

LabChip GXII Touch HT Protein Characterization System
Featured resources
Questions?
We're here to help.
Contact us Revvity is a trademark of Revvity, Inc. All other trademarks are the property of their respective owners.