Sole collaboration to UK Vaccine Task Force
The Certimmune ELISPOT was selected as the sole collaborator to the UK Vaccine Task Force (VTF) to assess T cell responses to vaccines, vaccine dosing strategies and prime-boost regimes.
The Certimmune ELISPOT was selected as the sole collaborator to the UK Vaccine Task Force (VTF) to assess T cell responses to vaccines, vaccine dosing strategies and prime-boost regimes.

26,000 ELISPOT and flow cytometry clinical trial samples
We completed ELISPOT and flow cytometry work on over 26,000 clinical trial samples in 2021.
We completed ELISPOT and flow cytometry work on over 26,000 clinical trial samples in 2021.
Global collaborations
We have collaborated with many companies, research institutes and governmental bodies in their clinical research.
We have collaborated with many companies, research institutes and governmental bodies in their clinical research.
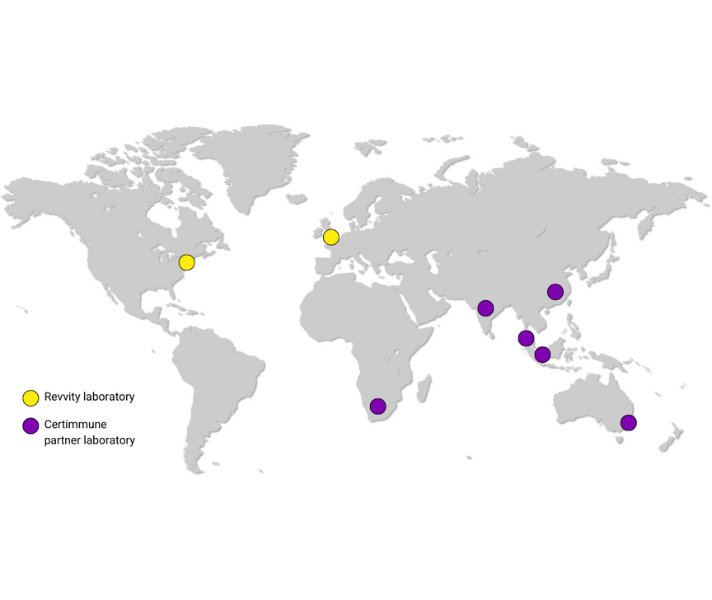
Global scalability for clinical trial growth
We have an established global network of trusted laboratories to provide testing services for our Certimmune customers. This is enhanced by use of T cell stabilization technology, which allows sample collection across greater distances.
We have an established global network of trusted laboratories to provide testing services for our Certimmune customers. This is enhanced by use of T cell stabilization technology, which allows sample collection across greater distances.
High standards to support regulatory submission
Guided by our Quality Assurance, Clinical Affairs, and Regulatory teams, our products and services adhere to GCP as standard in our labs.
Guided by our Quality Assurance, Clinical Affairs, and Regulatory teams, our products and services adhere to GCP as standard in our labs.
Our global reach
Featured resources
Questions?
We’re here to help.
Contact us For research use only. Not for use in diagnostic procedures.