Microfluidic Protein Characterization
Increase your protein characterization throughput
Proteins differ from each other in their size, molecular structure, and physiochemical properties. These differences allow for protein analysis and characterization by separation and identification. The LabChip® GXII Touch™ protein characterization system offers rapid peak quantification and quality control throughout the protein purification workflow.
Analysis can be performed in as few as forty seconds per sample that deliver comparable data to traditional capillary electrophoresis with as much as a 70X increase in throughput. Choose 96-well or up to 384-well platforms depending upon throughput needs. With an easy-to-use touch screen interface, users can get up and running samples quickly.
Supports FDA 21 CFR part 11 compliance
LabChip GXII Touch and Reviewer Software contain built-in technical controls and features specifically designed to support FDA 21 CFR Part 11 regulations. These features include a shared user account database, access controls, device check, enforced sequencing of run steps, audit trails, record copying, record retention, system documentation, and electronic signature controls.
LabChip GX reviewer - ProteinEXact
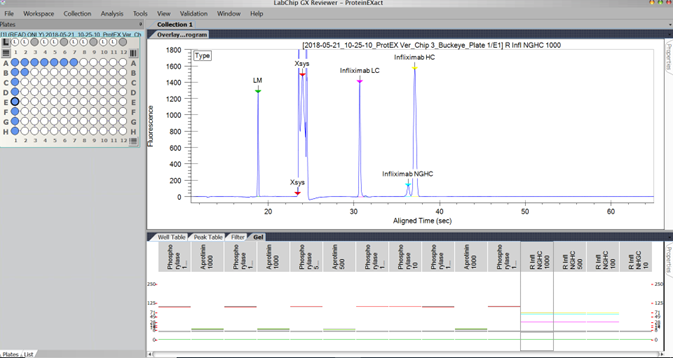
Learn how you can improve your protein characterization with the solutions below.
Analyze critical quality attributes in as few as 42 seconds including:
- Titer (concentration and sizing)
- Purity assessment
- Aggregation (covalently linked)
- Stability (degradation and fragmentation analysis)
- N-glycan profiling
- mAb charge variant and charge heterogeneity
- mAb impurity analysis (detect minor impurities)
For research use only. Not for use in diagnostic procedures.
Featured resources


Filters
1 - 25 of 26 Resources
Achieving higher throughput for the bioanalysis of advanced biotherapeutics
As advanced biotherapeutics require characterization and stringent quality control to ensure the best molecules are taken forward into the clinic, there's a continued need to assess critical quality attributes early on during the cell line development process. This whitepaper explores some of the technologies and solutions, from expression platforms to microfluidic protein characterization systems, that can be applied to save you time and money to fast-track the cell line development process, whilst ensuring biologics are produce with the required critical quality attributes. Covering areas including: CHOSOURCE expression platform Assessing N-glycan profiles of antibodies Expression of bispecific antibodies with transposon technology High-throughput characterization of bispecific antibodies For research use only. Not for use in diagnostic procedures.
Addressing the challenges in modern biotherapeutic development
Charter a course to biotherapeutic antibody development success The rise of bispecific antibodies for use as groundbreaking therapies continues to grow with more than 100 bispecific antibodies currently in development. This is thanks to their remarkable versatility from having dual binding sites targeting two different epitopes, providing advantages such as reducing resistance issues, increased specificity, and identifying unique combinations of drug targets. However, navigating the intricate challenges of bispecific antibodies is no small feat. That’s why we’ve created this infographic that will aid you on the journey, equipping you with the knowledge to overcome the obstacles along the way and achieve excellence in bispecific antibody development. Learn more about: Stages that make up the bispecific antibody path Challenges and considerations faced in development Various solutions and technologies available For research use only. Not for use in diagnostic procedures.
Addressing the challenges of bispecific antibody characterization with high-throughput platforms
Bispecific antibodies (bsAbs) have emerged as an attractive class of therapeutic agents with the potential to revolutionize the treatment of various disorders. While the concept of bsAbs has been around for several decades, it has taken time for the technology and understanding of antibody engineering to advance to a point where its practical application has become feasible. The development of bsAbs demands intricate screening processes to identify candidates with desired binding properties, ensuring they induce specific biological activities. Additionally, achieving optimal tissue tropism and potency requires careful consideration of factors such as antibody size, affinity, and the implementation of innovative characterization strategies. In this context, this whitepaper explores novel approaches contributing to bsAb characterization, offering real-world examples that highlight their practical applications.
An evaluation of control analysis system performance between the LabChip GXII and SCIEX PA800 Plus system
In this application note mAbs were evaluated using both the Protein Clear ™ HR assay and an orthogonal method, the SCIEX ® PA800 Plus system.
Automated characterization of proteins and nucleic acids
Read the LabChip ® GXII Touch ™ protein characterization system product note to learn about an automated alternative to traditional methods which streamlines slab gel electrophoresis, while providing increased throughput and data formats required for biotherapeutics and genomics workflows. This flexible instrument supports multiple assays for characterizing both proteins and nucleic acids.
Automated integrity assessment of RNA samples.
LabChip® RNA analysis provides a fast and easy way to quantitate RNA samples ranging in size from 100 to 6,000.
Automated microfluidic capillary electrophoresis for measurement of full and empty adeno‑associated virus capsids.
In this application note we describe a microfluidic capillary electrophoresis method that uses the LabChip® GXII Touch™ Protein Characterization System for AAV empty/full analysis.
Biologics workflow solutions
Precision biologics are playing an increasingly powerful role as part of therapeutic strategies such as monoclonal antibodies, bispecific and multispecific antibodies, recombinant proteins, vaccines, and targeted next-generation cell and gene therapies. Harnessing large molecules to create safe and effective therapies is a complex and expensive undertaking. So we help scientists like you develop and streamline your entire biologics workflow, helping you overcome the challenges to bringing consistent, high quality, biological medicines to market – faster than ever before. Explore our solutions and technologies that cover areas of: Biomolecular discovery Biologics characterization Investigating immune function Manufacturing and QA/QC Safety and efficacy
Cell line development solutions flyer
Redefining your workflow with Revvity cell line development solutions that help streamline your processes, improve productivity, and accelerate pipelines. Introducing Revvity’s CHOSOURCE ™ platform for cell line development workflows, from expression systems through cell health evaluation and protein quantification and characterization, and Revvity’s cell counting and imaging platforms for high-throughput image cytometry.
Characterization of NISTmAb with microfluidic CE-SDS
Capillary electrophoresis (CE), more specifically CE-SDS, is becoming an increasingly popular and routinely used method for monitoring and documenting critical quality attributes for monoclonal antibodies, due to its automated operation, on-column detection, resolving power, and protein quantification capability. Such characterization is frequently used in the development and manufacture of biopharmaceuticals. The LabChip ® GXII Touch ™ Protein Characterization System features microfluidic CE-SDS (µCE-SDS) technology for analytical characterization of monoclonal antibodies. It offers an automated alternative to traditional SDS-PAGE methods, by streamlining the multiple, manual steps of slab gel electrophoresis, while providing the throughput and data quality essential to biotherapeutic workflows. In this application note, the characterization of a reference monoclonal antibody from the National Institute of Standards and Technology (NIST) using the LabChip GXII Touch system is compared to those published by NIST using the SCIEX ® PA-800 Plus system.
Complementary platforms that enable multiparametric characterization of AAV capsids
Over time, Adeno-associated virus (AAV) vectors have become the leading platform for gene delivery in the potential treatment of a variety of human diseases. Researchers and bioanalytical scientists within the gene therapy community have focused on characterizing various AAV serotypes using conventional and next-generation technologies to deliver critical quality attributes along their workflow. In this application note, discover how AlphaLISA ™ and LabChip ® GXII Touch ™ microfluidics-based CE technology can be used as orthogonal methodologies to detect and characterize AAV8 serotype capsid proteins.
Compliance Made Easy Navigating FDA 21CFR Part 11
To aid in your compliance planning, we provide enhanced security options with the LabChip GXII Touch Protein Characterization System, encompassing access security, data security and verification, and a comprehensive set of audit logging functionality.
Harnessing cell line engineering to enhance biotherapeutics
Monoclonal antibodies (mAbs) are now one of the leading classes of biotherapeutics, with total sales expected to surpass hundreds of billions of US dollars in the next year. They also comprise over fifty percent of first-time regulatory approvals. To further enhance the therapeutic properties and efficacy of mAbs, researchers are focusing on developing more optimized methods and engineering processes through cell line selection and genetic modifications. In this whitepaper explore the potential of next-generation genome editing tools for helping drive the success of monoclonal antibodies as biotherapeutics. Along with the considerations to take when designing more potent therapeutic antibodies. Areas covered include: Choosing the right cell line Why post-translational modifications matter Afucosylation benefits Altering glycan composition through gene editing
High Throughput assays to compare the performance and stability of an engineered Fc silent antibody to a therapeutic antibody
Over the last decade, therapeutic antibodies have emerged as the predominant class of new drugs. Assays to functionally characterize and evaluate antibodies can be used in the discovery process as well as during the development stages of antibody therapeutics. In this detailed case study, we show a suite of assays that can be used to compare an engineered antibody (adalimumab Fc Silent ™ ) to the original therapeutic antibody (Adalimumab). In this application note, discover how Alpha, HTRF and LabChip ® Protein Express assays can help when you are: Comparing the binding affinities of engineered antibody or original therapeutic antibody to the target or Fc receptors, or for readouts in functional cell-based assays Assessing binding capabilities after thermal stress, to check antibody stability Looking for critical data to build confidence in a biosimilar or an engineered therapeutic antibody
High-throughput, end-to-end Cell Free DNA (cfDNA) analysis workflow from plasma
Benefit of an automated cfDNA analysis workflow The presence of double stranded, circulating cell free DNA (cfDNA) in blood plasma was discovered more than 70 years ago. Soon after, a link between cfDNA and leukemia as well as autoimmune disease was found. Recently, the development of cfDNA-based prenatal genetic testing and the realization that cfDNA can be used to detect and monitor tumor specific mutations in cancer patients has increased the interest in cfDNA analyses. Read the application note to review the results and how much time you could save by automating your cfDNA analysis workflow using Revvity’s automated solution including blood fractionation, cfDNA isolation, library prep, QC, and quantitation.
LabChip Empower Driver Flyer
The LabChip Empower Driver Flyer describes how you can take full control of your nucleic acid and protein assays on the LabChip GX Touch™ and LabChip GXII Touch™ systems with Waters Empower™ software.
LabChip GX Touch platform applications in epigenetic studies
The LabChip GX Touch nucleic acid analyzer is an important component of Oxford BioDynamics research in epigenetic biomarkers of disease and their viability for disease detection, prognostic testing, and drug screening for the development of personalized treatment plans.
LabChip GXII microcapillary electrophoresis to study primary proteolysis during cheese ripening.
The LabChip™ GXII Touch™ system provides a detailed and efficient method for studying primary proteolysis in cheese, helping improve control over cheese texture and flavor properties.
LabChip GXII Touch small RNA assay
The LabChip ® Small RNA assay was developed for analysis of small RNAs 20-150 nucleotides (nt) long. Together with LabChip ® microfluidic devices, the new LabChip® Small RNA assay allows users to separate and measure small RNA molecules easily, quickly, and reliably. Minimum sample consumption, re-useable microfluidics chips, and automated workflow make the high-throughput quality control screening of small RNA molecules more productively and cost effectively in modern genomic research and biotherapeutic development process. The assay provides sizing, concentration, and percent purity for small RNA analytes. The new gel formulation achieves resolution of 5 nt for 20-100 nt range, and 10% size difference for 100-150 nt. This allows users to check for degradation and confirm correct RNA size prior to using synthetic RNA molecules in expensive protocols such as CRISPR-Cas 9 workflows.
Mouse tail genotyping: A LabChip application to assess the genetic modifications on mutant mouse populations.
This application note describes how researchers at two organizations utilized Revvity workflows for genotyping reaction setup and microfluidic-based CE for PCR fragment analysis. This combination of automated solutions dramatically improved their experimental turn-around times and data quality while also reducing hands-on time and labor costs.
Overcoming challenges in liquid biopsy
This application note describes how the LabChip GX Touch Nucleic acid analyzer can be used with the nd the Agena MassARRAY® UltraSEEK™ Lung Panel. The LabChip GX Touch Nucleic acid analyzer optimizes pre-analytical factors relating to the collection, processing and extraction of cfDNA.
Safety rules with Revvity biotherapeutic workflows
Helping you put safety and product efficacy at the forefront of your GMP manufacturing At Revvity we’re able to support your GMP manufacturing workflows in areas such as detecting and quantifying harmful impurities along with helping you produce products that have both improved reliability and reproducibility. In this infographic you’ll get to grips with the instruments, reagents and assays that can help you produce safer and more efficient biotherapeutics. Covering areas of: Cell line development Screening and characterization Detection and quantification Meeting compliance requirements With it all backed by technical expertise and support, you’re biotherapeutic manufacturing just got a lot safer. For research use only. Not for use in diagnostic procedures.
Shifting the treatment paradigm: the promise of gene therapy for neurodegenerative diseases
Neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, and Huntington’s, are complex disorders that affect millions worldwide. Traditional treatments focus on managing symptoms, but recent advancements in gene therapy offer hope for more effective and lasting solutions. Key insights: Understanding neurodegenerative diseases: Learn about the prevalence, impact, and current treatment limitations neurodegenerative diseases. Gene therapy mechanisms: Explore how gene therapy works, including CRISPR gene editing, RNA interference, and other cutting-edge techniques. Approved therapies: Get detailed information on the four FDA-approved gene therapies for neurodegenerative diseases, including their mechanisms and delivery methods. Future directions: Discover the latest advancements in gene editing technologies and delivery systems that are paving the way for more effective treatments. Why Gene Therapy? Gene therapy represents a revolutionary approach to treating neurodegenerative diseases by targeting the root causes at the genetic level. This white paper delves into the science behind these therapies and their potential to transform patient outcomes. Exclusive Content: Detailed explanations of genome and RNA editing techniques. Insights into the latest delivery methods for gene therapy. Case studies of approved gene therapies and their impact. Future trends and research directions in the field. Fill out the form below to access the complete white paper and stay updated with the latest research and developments. For research use only. Not for use in diagnostic procedures.
Streamlining the development of bispecific antibodies from expression to quality assessment with Revvity’s biotherapeutic workflow solutions
In this application note we show how the CHOSOURCE ™ TnT Transposon Technology was used to express an asymmetric 4-chain bispecific antibody in the CHOSOURCE GS KO host cell line. Additionally, Revvity’s LabChip GXII Touch HT system is used to rapidly characterize the expressed proteins, showing that in combination with CHOSOURCE, biotherapeutic workflows can be streamlined and accelerated.





























