Since 2009, Revvity has been at the forefront of developing cell line-derived reference standards, continuously expanding our portfolio to empower researchers in their oncology research endeavors. In this blog post, we'll delve into the extensive process behind our Mimix™ reference standards and explore how these molecular tools are facilitating cancer research.
What are biological reference standards and what are they used for?
Real patient samples are often limited in quantity, can vary in quality and genetic profile. Reference standards offer researchers a material with known and fixed parameters that can provide a stable result to verify a successful experiment and to test for identity, concentration, and quality of a particular substance.
Reference standards can provide confidence in results and help identify and mitigate problems in research workflows by:
- Confirming that research assays are performing as expected
- Determining limits of detection and Minimal Residual Disease (MRD) status
- Enhancing quality during assay development and manufacturing
- Verifying end-to-end workflows with confidence
- Benefiting from robust routine monitoring and ease of troubleshooting.
How are Mimix reference standards generated?
Mimix reference standards are generated from a blend of human derived cell-lines with well characterized genetic variations such as insertions and deletions (INDELs), fusions, single nucleotide variants (SNVs) and copy number variants (CNVs). Because Mimix reference standards are cell line-derived, they maintain genomic complexity leading to more realistic results, comparable with actual patient samples. These reference materials are made using variants from existing cell lines harboring the variant of interest and/or by engineering desired mutations into cell lines using genetic engineering techniques such as rAAV and CRISPR. Cell lines are blended to create variants at specified frequencies then offered in various formats to mimic patient samples and for use in a variety of assays.
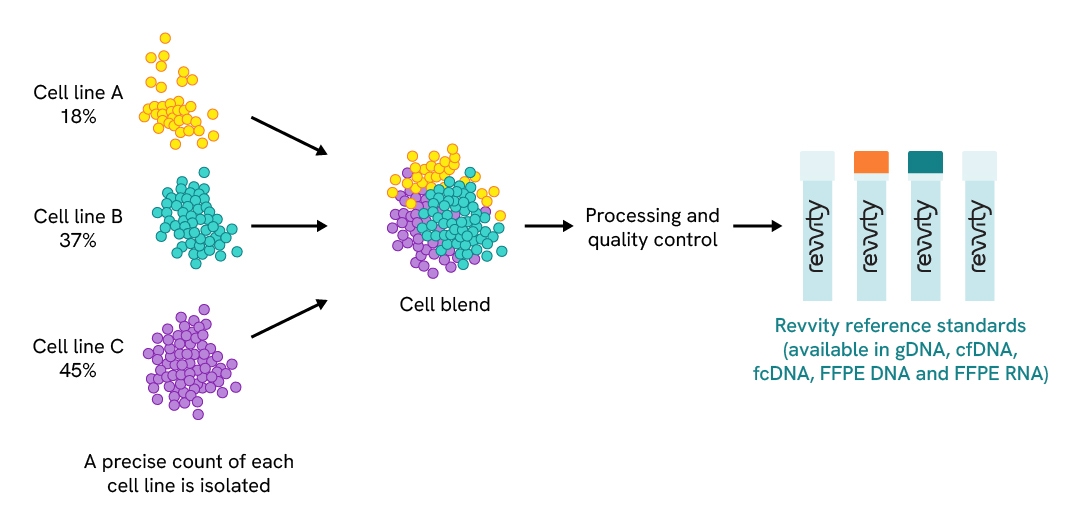
This unique cell line blend allows Mimix reference standards to mimic the heterogeneity of patient samples, allowing them to be more biologically-relevant. The cell blending strategy for Mimix reference standards is illustrated in Figure 1.

Blending cell lines to achieve clinical genomic complexity.
Figure 1: Characterized cell lines with variants are blended to create reference standards with a few or multiple variants of known allelic frequencies which then undergo quality control analysis to be available in a variety of formats.
How are Mimix reference standards applicable to a range of research requirements?
Mimix reference standards are available in variants with varying allelic frequencies that mimic the complexity of biological samples. Typically, an allele can be found at 50% or 100% allelic frequency depending on whether the allele is heterozygous or homozygous in a cell line. Blending a variant cell line with additional cell lines that harbor different genetically engineered or endogenous mutations generates reference standards with different allelic frequencies starting from 0.5%. By using wild type reference materials, the allelic frequencies can be brought down further for testing some assay specifically designed to detect very low allelic frequencies (MRD).
How can Mimix reference standards be applied to research workflows?
Mimix reference standards closely replicate patient samples throughout the research workflow, enabling researchers to verify results and more easily identify issues and failures. By using Mimix reference standards alongside patient samples, researchers can simulate the same processes as with actual samples, allowing for direct comparison of results. This approach provides reassurance for tests and workflows, as illustrated in Figure 2.
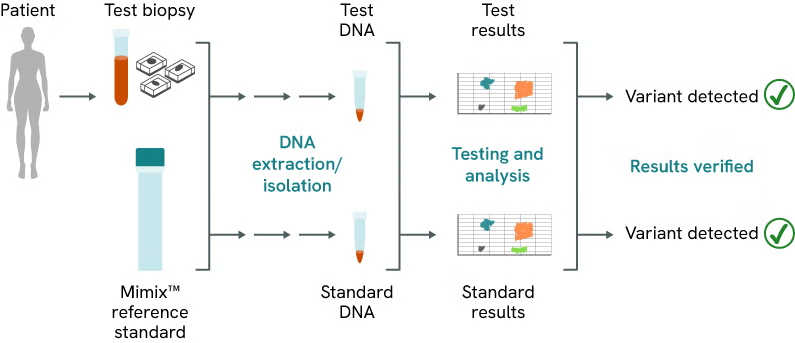
How standards mimic research workflows
Figure 2: Reference standards mimic an individual sample throughout the research workflow to act as a control for the assay/process. A reference standard can be used just for part of the assay testing or serve as an entire process control to bring verification to the entire workflow.
What formats are Mimix reference standards available in?
To ensure that Mimix reference standards are applicable to a range of cancer research workflows, Revvity offers a variety of formats with a range of genetic variants to allow researchers to find the best process control for their research workflows.
|
Reference standard formats |
|
|---|---|
| gDNA | Genomic DNA from cell lysates |
| cfDNA (ctDNA) | Cell-free DNA in Buffer or synthetic plasma |
| fcDNA | Formalin Compromised DNA |
| FFPE DNA | Formalin-Fixed and Paraffin-Embedded DNA |
| FFPE RNA | Formalin-Fixed and Paraffin-Embedded RNA |
Table 1: The range of Mimix reference standards product formats offered by Revvity. Mimix reference standards can be used as a technical control for the assay or as a process control for a workflow (Figure 3).
All Mimix reference standards are cell line-derived regardless of the product format to provide researchers with reliable and commutable controls that resemble real patient samples. Mimix reference standards offers both singleplex reference standards, that allow control for a single allele/variant of interest, and multiplex reference standards that can act as a control for multiple alleles/variants at differing allelic frequencies. Complex characterized reference standards at varying allelic frequencies allow researchers to know the limits of detection of their assay, have confidence in their results, and since multiple controls can be combined using our multiplex standards, they allow labs to run more samples.
Why are Mimix reference standards available in different formats?
Mimix reference standards are available in a wide range of formats allowing them to be applied to the full research workflow. Figure 3 shows how reference standards equip researchers with the ability to find the best process controls for their application from sample extraction through to assay development and bioinformatic controls.
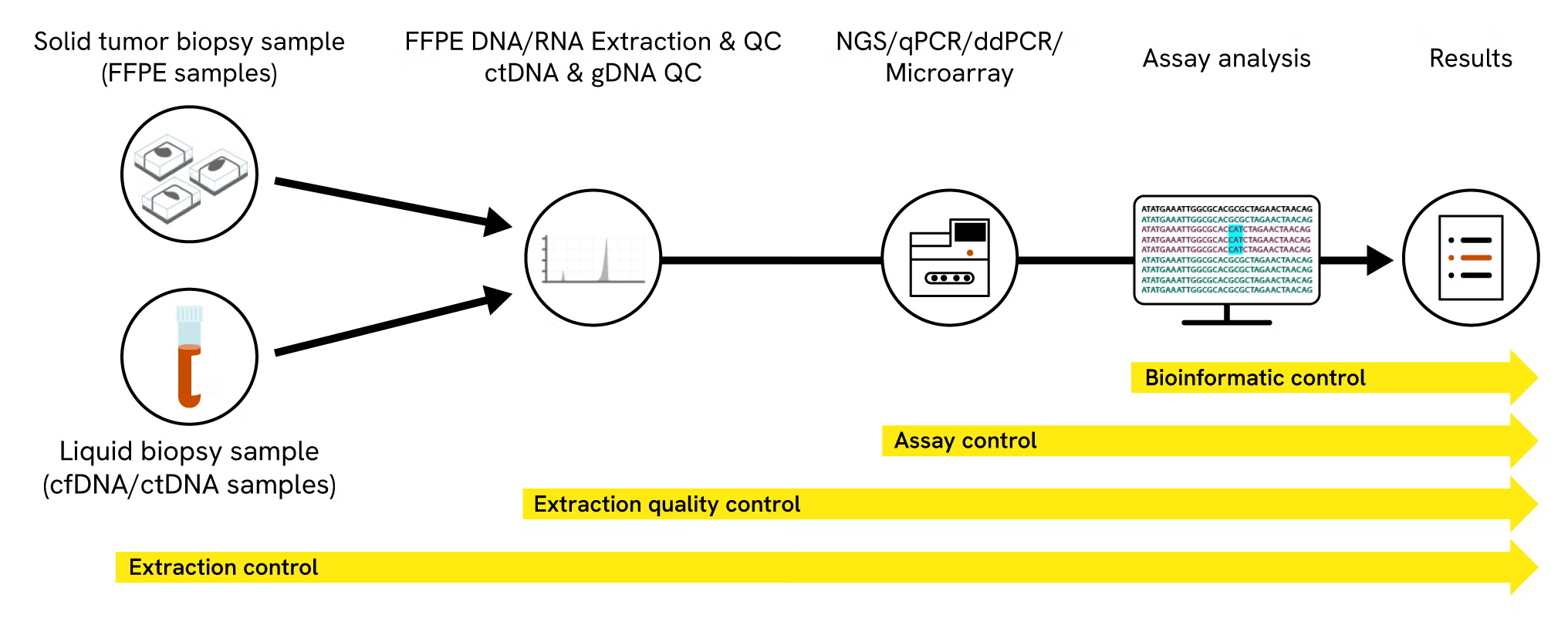
Standards for different parts of research workflows
Figure 3: Applying different reference standards to act as a control for different parts of a research workflow.
What happens if Mimix reference standards are not available in the required genetic variants of interest? We understand that cancer research is evolving constantly. To support nearly all research requirements, we also offer bespoke Mimix reference standards from Revvity’s in-house cell line engineering team. Select almost any genetic variants of interest and design reference standards tailored to your oncology research workflow in any format (FFPE/gDNA/ctDNA/RNA).
Create your own reference standard in five easy steps
How is batch-to-batch consistency maintained for Mimix reference standards?
Revvity performs stringent quality checks on each batch of Mimix reference standards so that variants are found at specific allelic frequencies by digital droplet PCR (ddPCR). The ddPCR analysis provides a consistent benchmark for oncology research. Detection of variants using different assays, such as NGS may vary based on many factors, some of which are discussed here.
Results from our analysis are provided online in the batch-specific Certificate of Analysis (CoA) found under the ‘Resources’ tab on each product page. The quantity and integrity of each reference standard is checked by quality control measures to deliver uncompromised genetic material (Table 2).
|
Reference standard quality control |
||
|---|---|---|
| Reference standard(s) | Quantification Method | Assay for integrity |
| gDNA | Spectrophotometry at A260 – Nanodrop | Agarose Gel Electrophoresis |
| cfDNA, fcDNA | Fluorescent DNA detection – Thermo Qubit® dsDNA BR (broad range) assay | TapeStation |
| FFPE DNA | Fluorescent DNA detection – Promega QuantiFluor® dsDNA assay | Agarose gel electrophoresis |
| FFPE RNA | Fluorescent RNA detection – Thermo Qubit® RNA HS assay | FFPE RNA tested for amplifiability by end-point RT-PCR or TapeStation |
Table 2: Our quality control process means that the Mimix reference standards provided are reliable genetic material to control your assay. Allelic frequencies are confirmed by ddPCR/NGS, and quantification and integrity are measured depending on the product type as noted in the table above.
Mimix reference standards are developed and manufactured under ISO 13485:2016 and ISO 9001:2015 quality management standards. This ensures consistency and reliability, and each batch of reference standards undergo thorough quality control testing to confirm the presence of specified variants.
It is possible that more variants may exist in a standard than those stated due to the complexity of a whole-genome product. A multiplex reference standard can act as multiple controls in one sample, freeing up more room for samples in an assay run. Our range of Mimix OncoSpan™ reference standards come with batch-specific NGS data available on purchase, allowing researchers to know other variants and backgrounds that are present in the standard.
Who can use Mimix reference standards?
Mimix reference standards can provide confidence in results, help to identify and mitigate problems within research workflows. Whilst reference standards are applicable in many applications, we work with some key groups including:
- Academic and clinical organizations
- Pharmaceutical companies
- External Quality Assessment (EQA) scheme laboratories
- Kit manufacturers.
By using reference standards, these organizations are able to verify the effectivity of their molecular assays and workflows. Mimix cell line-derived reference standards closely reflect results comparable to real patient samples which helps researchers to understand how assays will perform with actual samples when applied in real-world scenarios.
What platforms are Mimix reference standards compatible with?
Mimix oncology reference standards are platform-agnostic for implementation into quality control workflows and can be compatible across a range of platforms including Next Generation Sequencing, Sanger sequencing, droplet-digital and Real-Time PCR.
By applying biologically-relevant controls to the full research pipeline, issues and faults can be detected swiftly to deliver faster discovery and results. Mimix reference standards provide researchers with reliable, reproducible and affordable solutions to provide more confidence in research assays.
Learn more about the Revvity portfolio
Find out more about Mimix reference standards:
- The five quality control metrics every NGS user should know-Blog article
- Using reference standards in the development of new liquid biopsy platforms – Blog article
- Reference standard formats commutable with tumor biopsy and liquid biopsy samples for cancer genomic profiling – Poster
For research use only. Not for use in diagnostic procedures.










































