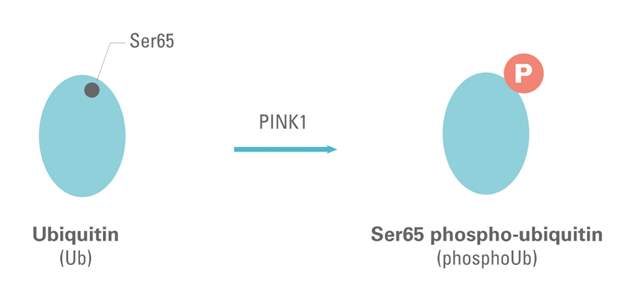
Ubiquitin (Ub) is covalently bound to many misfolded, denatured, or surplus cellular proteins and enables their degradation. It has been shown that PINK1 phosphorylates ubiquitin on Serine 65. [1] Furthermore, Ubiquitin phosphorylation at Serine 65 has been shown to be a specific marker of mitophagy activation.

Figure 1: Schematic representation of the phosphorylation of Ubiquitin on Ser65 by PINK1
Phospho-Ubiquitin Ser65 active PINK1/Parkin
In the case of stress or mitochondrial injury, PINK1 (protein kinase) accumulates specifically on depolarized mitochondria, while Parkin (ubiquitin ligase E3) catalyzes the transfer of ubiquitin to mitochondrial substrates. PINK1 has been shown to activate Parkin via two mechanisms. First, PINK1 phosphorylates ubiquitin on S65, competing with Parkin’s autoinhibitory domain which stabilizes in an active conformation. Then in the second step, PINK1 directly phosphorylates the ubiquitin domain on S65 of Parkin, inducing conformational changes which allow the binding of ligase E2. These two mechanisms increase the activity of Parkin’s ubiquitin ligase E3, inducing a complete activation of the PINK1/Parkin complex. Finally, Parkin amplifies the damage detection signal from PINK1, which increases Parkin recruitment and triggers mitophagy.
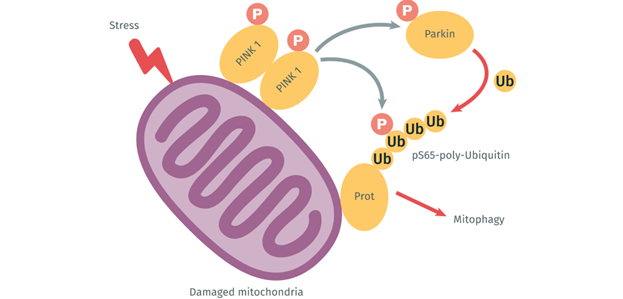
Figure 2: The activation process of PINK1/Parkin following the phosphorylation of Ubiquitin Ser65
Conclusion
Thus, ubiquitin is phosphorylated by PINK1 to activate Parkin, which triggers the mitophagy pathway. In contrast, mutations followed by the loss of function of PINK1 and Parkin lead to the early onset of Parkinson’s Disease. As well as this, alterations in mitophagy have also been associated with other neurodegenerative diseases, such as Alzheimer’s Disease or Amyotrophic Lateral Sclerosis.
Bibliography
- Tobias Wauer, Kirby N Swatek, Jane L Wagstaff.Ubiquitin Ser65 phosphorylation affects ubiquitin structure, chain assembly and hydrolysis.The EMbo Journal. EMBO J (2015)34:307-325.
- Preston Ge, Valina L. Dawson & Ted M. Dawson.PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson’s disease.Molecular Neurodegeneration. Article number: 20 (2020).
For research use only. Not for use in diagnostic procedures.