Impaired glucose consumption is one of the principal markers for many disease conditions such as cancer, diabetes, obesity, and some neurologic disorders. Positron emission tomography (PET) is the most commonly used technique for imaging and quantifying glucose uptake. It has significant drawbacks for laboratory use, however, namely the short half-life of the reagent, the need for an on-site cyclotron, potential exposure of personnel to ionizing radiation, and its high cost.
Maric et al. recently developed a novel bioluminescent glucose uptake probe (BiGluc) that enables non-invasive imaging and quantification of glucose uptake in vivo. BiGluc is highly sensitive, non-radioactive, and easy-to-use in diverse settings.
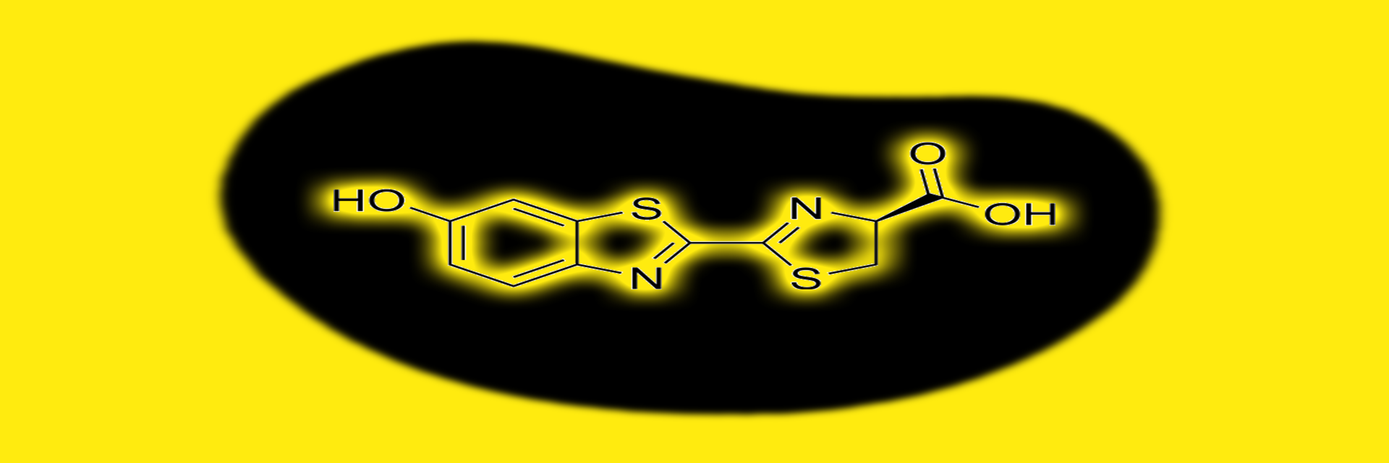
Design and synthesis
The Maric team approached the reagent design a biorthogonal click reaction between organic azide and the caged luciferin triarylphosphine. To overcome the slow kinetics of the ligation, they fine-tuned the azido-glucose derivative (GAz4) by introducing a perfluorophenyl azide to it. The modification increased the electron withdrawing properties of the molecule, leading to strong bioluminescent signal production.
In Vitro assays
The researchers conducted several in vitro assays using different cell lines to evaluate the BiGluc probe for imaging and quantifying D-glucose uptake.
- BiGluc light production was measured in three different cell lines that were stably transfected with luciferase under different conditions. The results identified concentration-dependent BiGluc signal reduction from D-glucose-treated cells, but no signal change in the non-natural L-glucose treated controls.
- The glucose transporter GLUT1 plays a critical role in the development of cancer. Thus, the dependence of BiGluc light output on GLUT1 activity was evaluated using CRISPR/Cas9-mediated GLUT1 gene knockout in 4T1 breast cancer cells that were stably transfected with luciferase to produce the GLUT1-deficient cell line. Comparison of signal outputs identified a direct correlation between light production and GLUT1-mediated glucose uptake. Additionally, the BiGluc probe measured a 40-50% reduction in glucose uptake by shRNA-mediated GLUT1 knockdown in 4T1-luc cells.
- Light output by BiGluc was measured in insulin-stimulated adipocytes. The results indicated significant increases in signal upon stimulation with insulin. The results also correlated with known PI3K-dependent insulin-induced glucose uptake, indicating the BiGluc probe is an effective means of measuring physiologically relevant processes in living cells.
- The effectiveness of BiGluc for measuring glucose uptake was directly compared to that of 3H-2DG and 2-NBDG, two other commonly used reagents. Insulin-treated C2C12-luc myotubes were incubated with the reagents, and the results demonstrated that BiGluc is just as reliable as 3H-2DG and more reliable than 2-NBDG. The data also indicate that BiGluc is more sensitive than GB2-Cy3 reagent as previously reported, likely due to the smaller modifications made to the D-glucose structure of GAz4 compared to that of GB2-Cy3.
In Vivo assays
The BiGluc probe was further evaluated by in vivo assays using transgenic reporter mice (FVB-luc+/+) with and without xenograft tumors.
Competition studies were conducted using high concentrations of D-glucose and insulin stimulation in transgenic reporter mice. The results confirmed the selectivity of BiGluc for D-glucose uptake.
BiGluc was evaluated for applicability to tumor models using mice bearing xenograft tumors of 4T1-luc and 4T1-luc GLUT1 knockouts (4T1-luc GLUT1-/- #1). BiGluc signal production significantly decreased in GLUT1-knockout mice compared to 4T1-luc controls.
The appropriateness of the BiGluc probe for identifying novel GLUT1 inhibitors was also evaluated. Mice with 4T1-luc breast cancer xenografts were treated with a small molecule inhibitor of GLUT1 that blocks glucose transport in cancer models. The BiGluc light output in treated mice was significantly lower than light output in vehicle-treated control mice. The results also indicated a direct correlation between light production and GLUT transporter activity.
The performance of BiGluc was compared to that of 18F-FDG-PET reagent in the same animal model. The results demonstrated identical performance by the reagents.
These studies demonstrate that the novel BiGluc probe can be a reliable, versatile, non-invasive tool for in vitro and in vivo studies of glucose uptake. Potential applications may include:
- Studies of glucose uptake in deep tissues.
- Monitoring the development and progression of diseases linked to aberrant glucose uptake.
- Reliably measuring glucose uptake in various animal disease models.
- Screening GLUT inhibitors in drug development.
Reference
- Maric, T., Mikhaylov, G., Khodakivskyi, P., Bazhin, A., Sinisi, R., Bonhoure, N., Yevtodiyenko, A., Jones, A., Muhunthan, V., Abdelhady, G., Shackelford, D., & Goun, E. (2019). Bioluminescent-based imaging and quantification of glucose uptake in vivo. Nature methods, 16(6), 526–532. https://doi.org/10.1038/s41592-019-0421-z
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.