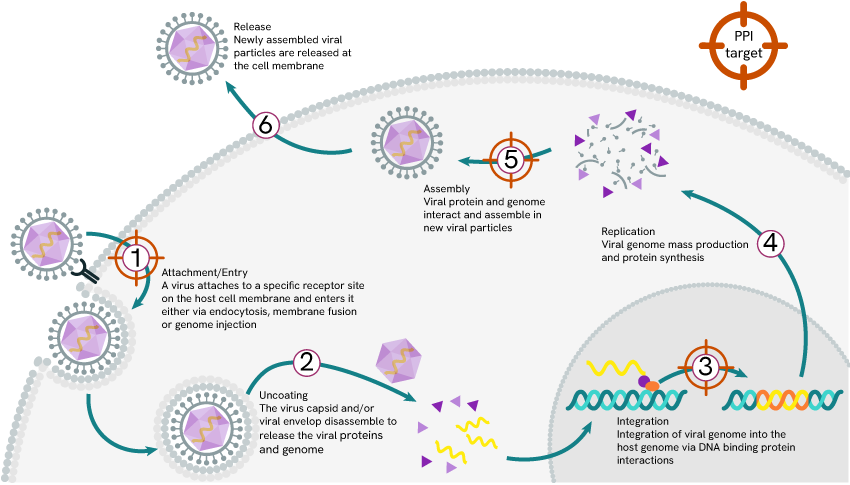
Therapeutic approaches to virology base themselves on strategies that aim to disrupt the viral lifecycle. As shown below, several steps in this cycle are protein-protein interaction events (PPI) in which a virus sequentially binds its way into the cytoplasm and nucleus of a host, interacts with DNA to add its own to the code and aggregates its newly synthetized components into fresh viral particles before binding its way out.

Protein-protein interaction targets in the viral life cycle
Consequently, building upon the advances in fluorescence and detection technologies among which tr-FRET, protein-protein interaction research and assay development were entered by significant players over the last decade has provided valuable results to the quest for better viral understanding and therapies.
Attachment
Among the pharmaceutical giants, MedImmune showed an interest for the field in 2012 and developed a platform suited for the study of live HRV16 in a virus/host cell attachment context. Focusing on the ICAM-1 binding strategy of HRV16, they used the mAb Anti-FLAG M2-XL665 and the europium cryptate labelling kit to create a format that identifies inhibitors of this viral attachment step. Putting their assay to use, they were able to successfully identify a collection of single-chain variable fragments and antibodies exhibiting the desired virus blockade potential. (Ref 1)
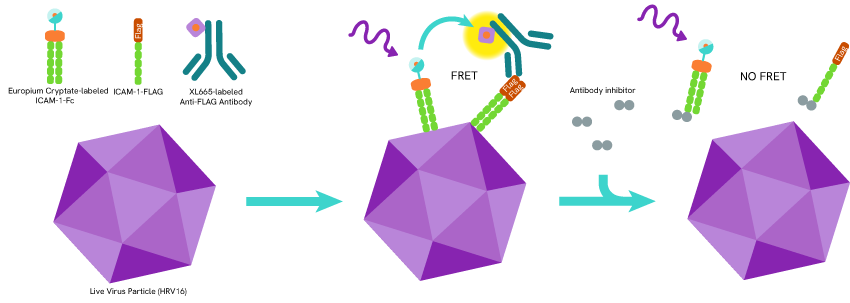
Virus blockade assay principle: ICAM1-Fc and ICAM1 labeled with Europium cryptate (donor) and Flag peptide respectively are incubated with live HRV particles and anti- Flag antibodies coupled with XL665 (acceptor). The virus- CAM1/ICAM1-Fc binding brings the Europium cryptate (donor) and XL665 (acceptor) in proximity and triggers FRET. The addition of anti-ICAM1 antibodies inhibits HRV/ICAM1 binding and shuts off FRET.
Integration
Prior to this discovery, Gilead had investigated the integration of HIV-1 genetic material into its host DNA and found that preventing the viral integrin dimerization ruptured its interaction with the host cell Lens Epithelium Derived Growth Factor (LEDGF), which considerably decreased the efficacy of DNA integration (antibodies: mAb Anti-6HIS XL665 and mAb Anti-FLAG M2-Eu crypate (Ref 2))
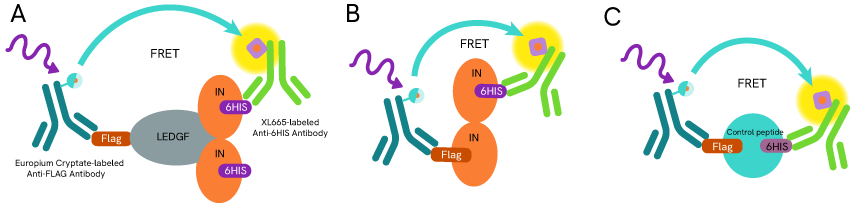
Viral DNA integration inhibitor screening assay principle: Anti-Flag and Anti-6HIS antibodies are coupled with Eu+ cryptate (donor) and XL665 (acceptor) respectively. (A) LEDGF and Integrase (IN) are labeled with Flag peptide and 6HIS respectively to monitor the interaction of Integrase dimer and LEDGF. (B) Two populations of Integrase are labeled with Flag peptide and 6HIS respectively to monitor the Integrase Dimer dimerization. (C) A control peptide is designed with and N-terminal 6HIS tag connected to a C-terminal Flag tag to identify potential positive inhibitors that would impair antibodies binding.
Assembly
Virology is not only attracting corporate interests, the field also draws curiosity from academic researchers, who have contributed to the research findings. Two striking examples sharing the same protein-protein interaction approach are the works of Kota et al (2010) and Thenin-houssier et al (2016), that rely on tagged proteins and associated anti-tag antibodies mAb Anti-GST-Eu cryptate and mAb Anti-FLAG M2-XL665. Both teams focused on viral assembly and implemented assays to screen for capsid protein dimerization inhibitors. Both studies were successful at identifying potential inhibitors of the assembly of the Hepatitis C virus and HIV-1 respectively. (Ref 3 and Ref 4)
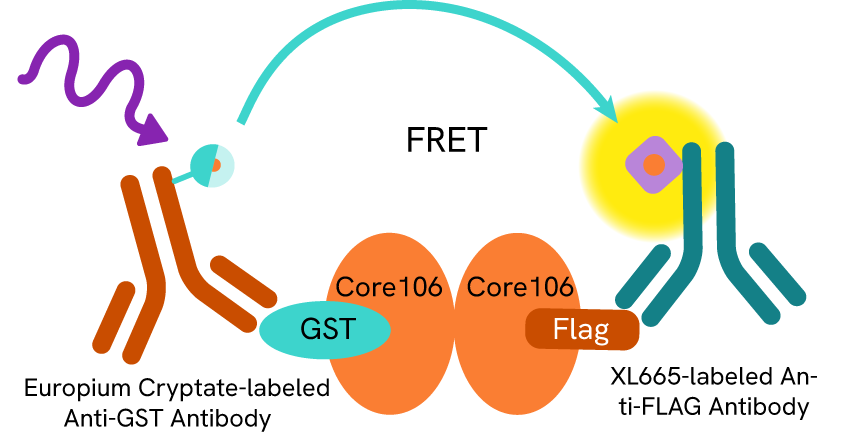
Viral capsid inhibition assay principle: Core proteins are tagged on the N-terminal domain 106 last amino acids with GST and Flag peptide respectively. Anti-GST and Anti-Flag antibodies are coupled with Eu+ cryptate (donor) and XL665 (acceptor) respectively. The dimerization of Core proteins via their 106 N-terminal domains brings the donor and acceptor-coupled antibodies in proximity and triggers FRET. The addition of tag-free Core106 domain creates competition and shuts off FRET.
Research around protein-protein interactions in the viral life cycle is varied and prolific. It stimulates the interest and curiosity of both the pharmaceutical industry and academic researchers, producing an array of knowledge and potential drug candidates over the last decade. Advancements in fluorescence-based detection and the increasing relevance of tr-FRET empower scientists with finer, more significant tools to tackle the virology studies. While viral infections and pathologies remain one of the great challenges of modern medicine, our understanding of them as well as our therapeutic arsenal is and will hopefully continue to grow stronger year over year.
For research use only. Not for use in diagnostic procedures.
- Ref 1: Development of a homogeneous high-throughput screening assay for biological inhibitors of human rhinovirus infection - PubMed (nih.gov)
- Ref 2 : Affinities between the binding partners of the HIV-1 integrase dimer-lens epithelium-derived growth factor (IN dimer-LEDGF) complex - PubMed (nih.gov)
- Ref 3 : A time-resolved fluorescence-resonance energy transfer assay for identifying inhibitors of hepatitis C virus core dimerization - PubMed (nih.gov)
- Ref 4 : Ebselen, a Small-Molecule Capsid Inhibitor of HIV-1 Replication - PubMed (nih.gov)