Newborn screening workflow
-
Newborn Screening Kits
Learn more
Revvity Transcribe AI

Revvity Transcribe AI, is an innovative OCR service designed to convert handwritten text on test request forms into a digitized format.
EVOYA Platform for Newborn Screening Software

Laboratory screening software that supports newborn screening workflows from sample collection to diagnosis and follow up offers numerous benefits for healthcare providers, researchers, and patients alike.
Sample preparation punchers
DELFIA Plateshake instrument
Incubators
Newborn Screening Kits
Use the highest quality reagents available today to screen for core newborn disorders as well as those detected by expanded screening.
Our reagents are the industry standard for analytical performance and reliability in detecting congenital diseases that are treatable only when identified during the first days of life.
Manual platform
Gel electrophoresis
All-in-one automation
Tandem MSMS
Molecular systems
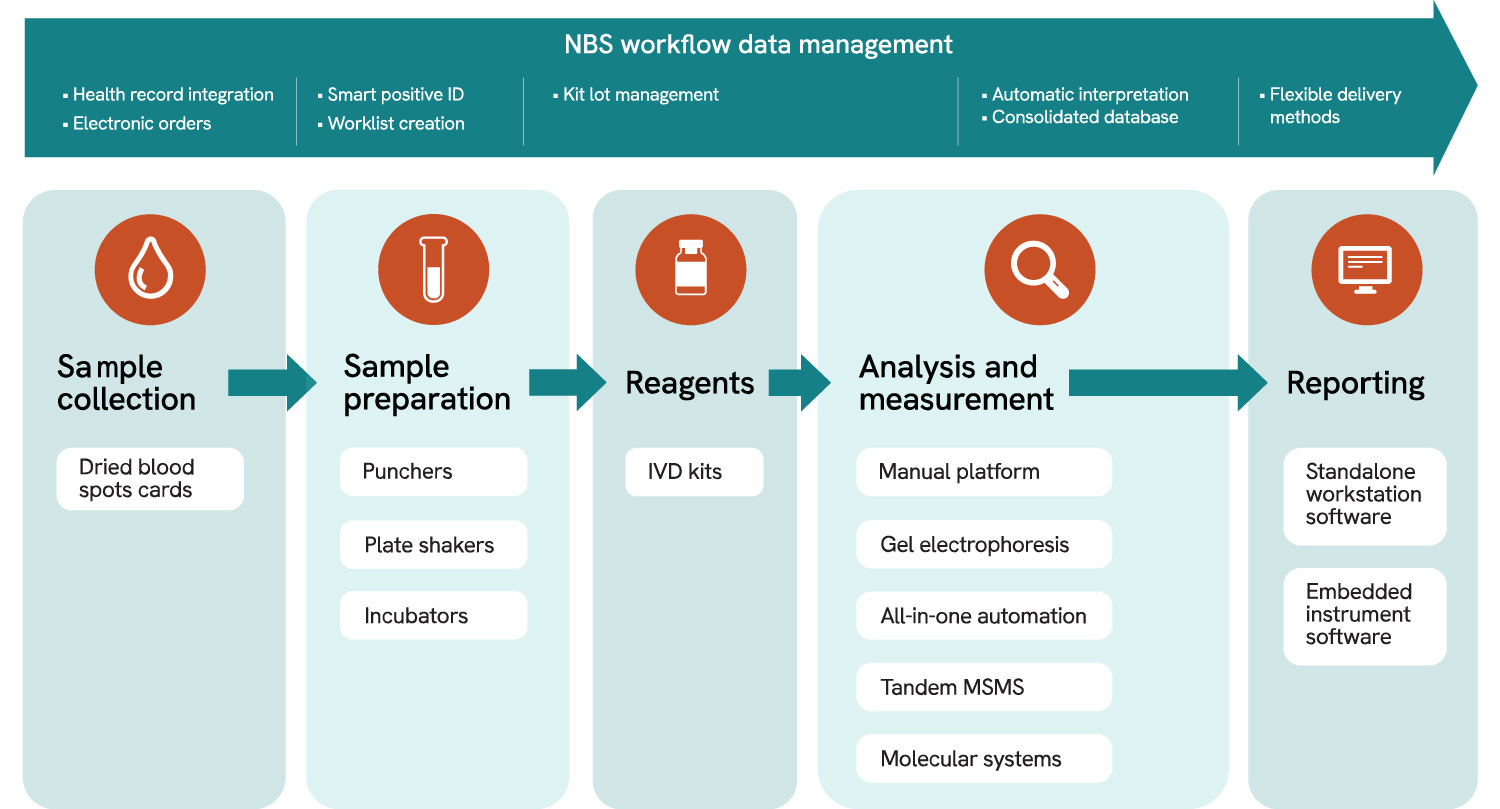
Reporting

Complete integrated workflow
Sample collection
It all starts here.
An accurate sample is the first step in ensuring quality results later in a newborn screening workflow. Our streamlined dried blood spot sample (DBS) collection cards are designed to support accurate and reliable results.
The cards can be printed to the specific format of your lab, offering an easy and effective method for simple and precise DBS collection.
Sample preparation
Consistent practices in sample preparation contribute to reliable and reproducible test results,1 whether you are looking to simplify and automate your sample prep or use real time visualization to optimize your punching, our preparation instruments will support you in building stronger workflow screening processes.

DELFIA Trio
DELFIA Trio™ combines disk removing, washing, and dispensing into one instrument offering a streamlined workflow for manual DELFIA users.

DBS Puncher Instrument
The DBS Puncher™ automatically punches dry blood spot samples into microtitration plates. The punch has a changeable head, so the blood spots may be 3.2 mm, 4.7 mm or 6 mm in diameter.
Two plates may be loaded simultaneously and the plate height allowed is adjustable, so deep well plates can be accommodated. After punching, disk detection ensures that the disk is correctly placed in the microtitration well.
The DBS Puncher may be connected to a bar code reader for indentification of the patient sample. It may be run from an external computer, receiving a plate layout which specifies the sample codes for the wells and the punch sequence.
The instrument is controlled by hand or by footswitch. Ease of use is enhanced by a clear double line display, and by illumination of the area being punched.

Panthera Puncher 9 Instrument
Revvity has selected the technical solutions and materials needed to assure a new standard of punching excellence in terms of:
- Efficient use of sample material
- More plates simultaneously
- Enhanced traceability
- Good ergonomics
Panthera-Puncher 9 automatically punches dry blood spot samples into microtitration plates. Up to 9 microtitration plates can be prepared simultaneously, and up to 3 of the plates can be deep-well plates. There are five different punch head sizes (diameters 1.5, 3.2, 3.8, 4.75 and 6.0 mm) and 2 punch heads may be installed at a time so plates requiring different blood spot sizes can be punched simultaneously.
Panthera-Puncher 9 includes a camera to provide a clear full-color view of the punching area in real-time, and an automatically adaptable punch pattern that adapts to the shape and size of the blood spot. This allows optimal use of the sample material without requiring the user to adjust the pattern to fit the area to be punched.
Specifications
Physical dimensions:`Width: 876mm (34.6''), Depth: 740mm (29.1"), Height: 560 mm (22.0") when the instrument's feet and the monitor are set to minimum height. The monitor can be adjusted up to 100 mm higher Weight: 45 kg (99 lb)
System: External PC, with Windows 7
Power requirements: Power consumption: 70 VA, Mains Voltage: 100-240 V, 50-60 Hz
Environmental conditions: Operating temperature: 15-30(102, 102, 102);°C (59-86°F), Relative humidity: 20-80 % for 3.2 mm punches and larger, 40 - 80 % for 1.5 mm punches
Performance Specifications
Real time visualization
- The instrument is equipped with a camera and a monitor, showing the sample card and the punching action in real-time and in color
- Easy positioning of the card
- Supports improved punching ergonomics
- Also the cassette/card barcode is read at the same time, with the same camera, in the same movement
- The user interface is controlled by a touch screen
Automatically adaptable punch pattern
- Punch locations are set according to the shape and size of the blood spot
- Limit values can be set for distance from the edge of blood and distance between the punches
- After its barcode is read, the card can be punched from either side
- If all queries cannot be processed using the same spot, the instrument will continue to process them from another spot on the same specimen card
- A fixed punch pattern may alternatively be set
- 1.5, 3.2, 3.8, 4.75, 6.0 mm punch sizes, two can be used in the same run
9 plate capacity
- 9 standard 96 well plates (ANSI SBS 1-4), with height 14 - 16.1 mm can be loaded in the instrument at the same time. Alternatively 3 of the available plate positions may be occupied by deep well plates, with max. height 44.5 mm, leaving 6 plate positions free for standard plates
- Significant improvements in the lab workflow of labs running more than 6 assays Punching 9 disks from one spot into different locations on 9 plates takes ~14sec
- Removal of static electricity by ionizer module in the punch area
Improved ergonomics
- Designed to allow more comfortable positioning of user's arms and shoulders
- The table may be raised or lowered electronically to suit the height of the user
- The height, distance and angle of the monitor can be adjusted.
Simplified user maintenance
- The user can remove the punch heads from the instrument, instructions shown in the user interface
- All covers are removable to support easy access for cleaning (wiping or vacuuming)

Trinest Incubator shaker instrument
TriNEST incubator shaker is a high-quality solution for all well plate applications, where accurate and fast temperature and/or shaking control are needed.
Reference
- Ellison, S.L.R., Hardcastle, W.A. Causes of error in analytical chemistry: results of a web-based survey of proficiency testing participants. Accred Qual Assur 17, 453–464 (2012). doi.org/10.1007/s00769-012-0894-2
Analysis and measurement
Our range of newborn screening instruments offer scalable, customizable, integrated solutions that sit across MSMS, Molecular, Immunoassay and IEF.
From manual to fully automated workflows
Designed to be easy to use and deliver fast, accurate results with quality and performance standards built in, ensuring strong equipment uptime.

VICTOR2 D Instrument
The VICTOR2 D fluorometer forms part of the semi-automatic DELFIA system, a cost effective alternative to the fully automated AutoDELFIA system for clinical or research labs. Other components of the semi-automatic system are the DELFIA Plateshake, DELFIA Platewash, and DELFIA Plate Dispense.

Migele Gel Electrophoresis Unit
Migele™ Gel Electrophoresis Unit works with the RESOLVE™ hemoglobin kit to detect hemoglobinopathies in newborns and adults, including sickle cell disease and other hemoglobin variants and thalassemias. Now, laboratories around the globe can conduct these crucial, lifesaving screenings with our easy-to-use, cost-efficient, scalable solution. The unit works with a water bath and a power supply to run the IEF gel. The recirculating water bath stabilizes the temperature of the gel during the IEF process, while the programmable power supply provides high voltage to the electrophoresis unit. When the gel is positioned in the unit and an electrical current is applied to the gel, the hemoglobin variant possessing an individual isoelectric point (pI) migrates through the gel. When an individual variant’s pI equals the pH in the gel, it stops migrating and forms a discrete band. When all hemoglobin bands have been focused, the gel is fixed in trichloroacetic acid. To ensure band visibility, we recommend that newborn blood spot samples be stained with our JB-2 staining system.

GSP Instrument (IVD)
The instrument is fully automated, performing every stage of an assay from retrieval of the sample plate from the stacker to measurement and reporting of results. Genetic Screening Processor utilizes specific GSP Workstation software for handling of the newborn screening data.
Design of GSP Instrument, software and screening assays offers multiple advantages for your screening program, including:
- Continuous loading of sample allowing more flexible workflows
- Barcoding of all materials to minimize risk of error and hands-on time
- Multi-technology solution to support high sensitivity DELFIA as well as prompt fluorescence measurements
- Extreme ease of use enabling quick user adoption and also rotation of staff duties
- Newborn screening specific informatics bringing the maximum operational efficiency
- Integrated screening operation supporting further growth and change of your program.

Eonis Q96
Eonis Q96 is a real-time PCR instrument with possibility to integrate into automation systems.
The Eonis Q instrument uses dried blood spot samples (DBS samples) punched from DBS cards. The DNA from the punches are extracted with a simple extraction protocol. No wash steps are required, only a 20 minute-incubation in a simple incubator.
Eonis Q PCR cycler, that includes a dedicated LIMS-compatible analysis software for the analysis and reporting of the results.

QSight MD Screening Systems (IVD)
With QSight® MD systems, we’re enabling expanded high-throughput mass spectrometry screening and, with the QSight 225 MD UHPLC Screening System, the option for tier 2 testing – using just a single heel-prick dried blood spot (DBS) card. The QSight system delivers a unique combination of self-cleaning MSMS instrumentation that provides exceptional system uptime, a simple setup process, and easy-to-use database software that simplifies review and analysis – plus kits and reagents, training, and support.
It all comes together to enable you to screen thousands of samples per day, every day.
Reagents
We build high-quality, reliable reagents that support accurate detection of congenital disorders from the first days of life.
Our comprehensive reagent portfolio covers 50+ different disorders and integrates beautifully with our instruments across the newborn screening workflow.
Newborn Screening Kits
Use the highest quality reagents available today to screen for core newborn disorders as well as those detected by expanded screening.
Software
Nothing is more important than ensuring newborn screening data remains safe and secure.
As the unifying thread that runs throughout a newborn screening workflow, software systems like EVOYA and OZ Systems integrate fully to help you manage your data more flexibly in the cloud. These solutions provide quality checks and controls whilst ensuring patient results are flagged properly for follow-up.
All this whilst keeping NBS data secure through security and compliance standards.
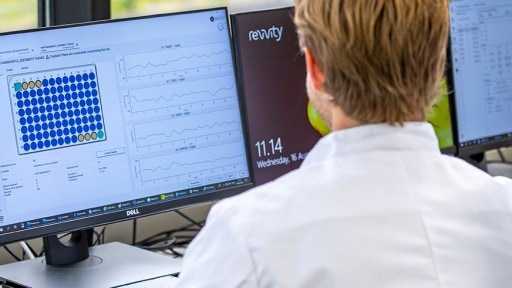
Newborn Screening Software
Control and monitor the entire newborn screening process – including patient reporting and follow-up -- in one comprehensive information management system.
Helping babies survive and thrive
Newborn screening can prevent hundreds of thousands of instances of physical and mental disabilities and even deaths. As the global leader in newborn screening, over 800 million babies have been screened with our products over the last 28 years.
50+ conditions
are known to have good outcomes when detected and diagnosed early
85 newborns
get a healthier start to life everyday thanks to screening using our products
Explore our newborn screening products


Revvity does not endorse or make recommendations with respect to research, medication, or treatments. All information presented is for informational purposes only and is not intended as medical advice. For country specific recommendations please consult your local health care professionals.
Products may not be licensed in accordance with the laws in all countries, such as the United States and Canada. Please check with your local representative for availability. Please note that product labeling (such as kit insert, product label, and kit box) may be different compared to the company branding. Please contact your local representative for further details.