
Yeast in biofuel overview
In general, yeast used in the biofuel industry are in complex medium such as corn mash, corn stover, or sugar cane, which can serve as nutrients to produce bioethanol. These complex media increase the difficulties for automated counting using brightfield method due to the debris particles in the sample. Using a combination of acridine orange (AO) and propidium iodide (PI) can perform dual fluorescence detection of live and dead cells, respectively, which can specifically stain the live/dead cells to reliably determine the concentration and viability of the complex sample.
Fluorescence based image cytometry method for yeast concentration & viability measurement

1. Pipette 20 µl of sample mix

2. Insert slide
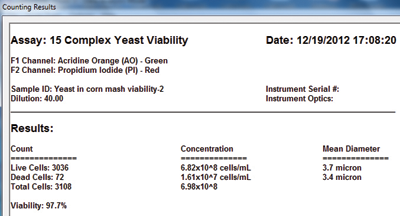
3. Click count and get results
How does yeast count & viability by dual fluorescence work?

A highly viscous corn mash sample is mixed with a dilution buffer and stained with nucleus staining dyes.
Live nucleated cells emit green fluorescence when excited by blue light.
Dead cells emit red light when excited by green light.
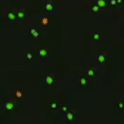
Live and dead cells are then distinguishable by color and viability is generated as a percentage based on live/total cell count.
Yeasts used in biofuel industry
Yeast concentration & viability measurement
Below shows 3 complex samples of corn mash, corn stover, and sugar cane. The brightfield images contain many debris particles, but in the fluorescent images, only the yeast cells that fluoresce brightly will be automatically counted.
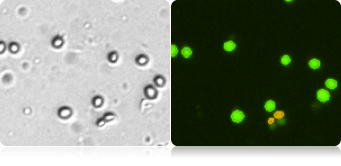
Corn mash debris
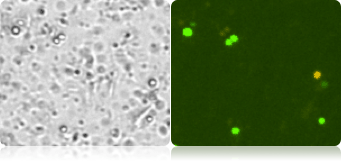
Corn stover debris
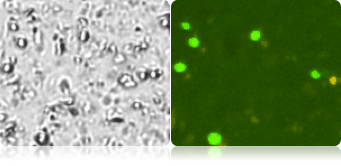
Measuring yeast viability using AOPI
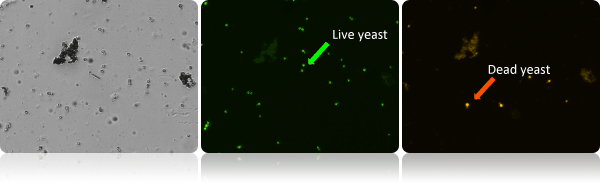
Identification of live and dead cells in a complex yeast sample
The images above show a brightfield, green (AO), and red (PI) images. Live cells in the green AO image are shown with a green arrow. Dead red PI positive cells are shown with a red arrow
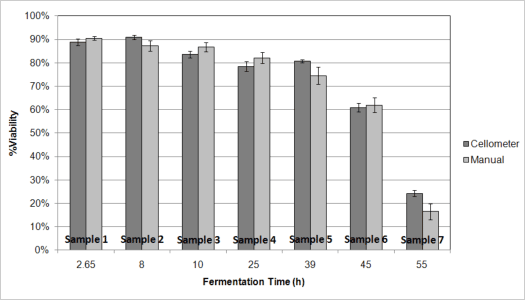
Multiple yeast samples were monitored during the ethanol fermentation process. All of the samples were counted using either the manual hemacytometer method or the automated Cellometer method. Early in fermentation the measured viability was shown to be at ~90 %. By the end of fermentation, at 55 hours, the measured viability was below 30 %. Both manual and automated methods showed great correlation to each other.
For research use only. Not for use in diagnostic procedures.






































