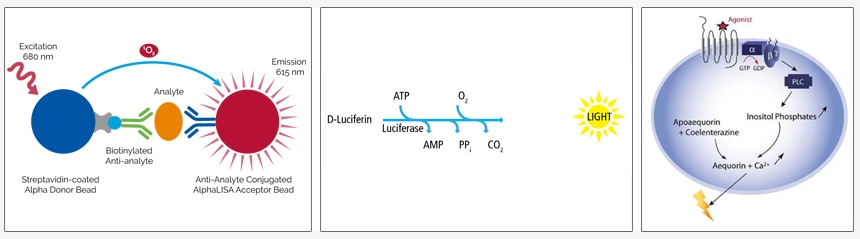
Overview
Luminescence-based assays are assays in which a luminescent signal (in the form of light, or photons) is generated via a chemical or biochemical reaction and measured using a plate reader. In general, light collected from luminescent assay measurements is not restricted to particular wavelengths. In most luminescent assays, signal from all of the photons produced by the assay is recorded by the PMT (photo-multiplier tube), CCD (charge coupled device), or other detector within the plate reader.
Examples of luminescent assays include:
- Alpha immunoassays - AlphaLISA™, AlphaScreen™, Alpha SureFire™ Ultra™
- Luciferase-based reporter gene assays – britelite™ plus, neolite™, steadylite™ plus, sensilite™, twinlite™
- Luciferase-based cytotoxicity and cell proliferation assays – ATPlite™, ATPlite™ 1step
- Calcium assays – AequoScreen™, PhotoScreen™
- Chemiluminescent ELISAs

Figure 1: Examples of luminescence-based assays. Left to right: AlphaLISA immunoassay, principle of luciferase-based reporter gene and cytotoxicity and cell proliferation assays, Aequorin assay for calcium flux.
White vs. Black vs. Gray Plates
When selecting a plate for a luminescent assay, it is important to consider whether you would like to use white plates, black plates or gray plates for your assay. There are strengths and weaknesses associated with each option, and certain types of assays may recommend or even require one particular color plate.
Signal
The use of white plates will result in a higher signal for luminescent assays, as they offer maximum reflection of light. Black plates can "quench" the signal by absorbing some of the light produced by the assay. Gray plates give an intermediate signal level. If you are working with an assay that produces a low signal, or if you are working in higher density format (1536-well plates), white plates may be helpful in maximizing signal. If you are working with an assay that gives a strong signal, black plates may be helpful in reducing well-to-well cross-talk.
Well-to-well cross-talk
Optical cross-talk occurs when light from one well travels through the well walls into adjacent wells where it is detected and adds non-specific counts to that well.
Phosphorescence and dark adaptation
Background phosphorescence may also be a consideration when choosing between white or black plates for a luminescent assay. Phosphorescence is the emission of light by a substance, resulting from stored energy. Certain components in a buffer or sample may phosphoresce, and plastic from the microplate itself can also phosphoresce. Phosphorescence can lead to increased background, which could potentially have a negative impact on a given assay. Black microplates intrinsically exhibit less phosphorescence than white microplates and may be desirable for particular assays. Alternatively, assays run in white microplates can be "dark-adapted" by shielding the microplate from light for up to 10 minutes prior to reading the plate in order to reduce background phosphorescence.
Gray plates
Certain plates are offered in black, white, or gray colors. Gray plates can be helpful as they can offer reduced cross-talk and reduced phosphorescence (in comparison to a white plate) while still maintaining high signal (in comparison to a black plate). Gray plates are designed to give low-background while maintaining high signal. This combination can result in higher sensitivity for some assays. For alpha assays including AlphaLISA™, AlphaLISA™ SureFire® Ultra™, and AlphaScreen™, we offer light gray AlphaPlate™ microplates, which are specially designed for low cross-talk in Alpha assays. AlphaPlate microplates can also be used for other types of luminescence assays such as ATPlite™ or other luciferase-based assays.

Figure 2: White 384-well OptiPlate™ microplate (left) and light gray 384-well AlphaPlate microplate (right).
Notes
- Alpha assays, including AlphaLISA™, AlphaLISA™ SureFire® Ultra™, and AlphaScreen, should never by run in black microplates. See more information on plate recommendations for Alpha assays further down in the page.
Microplates for cell-based luminescent assays
Choosing the correct microplate for a cell-based assay will be dependent on both the specific cell line being used in the assay and the assay protocol itself. Questions that need to be answered in choosing the plate include:
- Do I need a sterile, tissue culture-treated plate?
- Does the plate need to be coated?
- Should the plate have a clear or opaque bottom?
Plate considerations
Whether or not it is necessary to use a sterile, tissue culture-treated plate depends on the length of time the cells are going to be in the assay plate. In some assays the cells are added to the microplate and the assay is completed within a few minutes to a few hours. In other cases cells are grown in plates at least overnight prior to performing the assay, or are treated with compounds for extended lengths of time. As a general recommendation, if the assay is going to be performed within a single working day a sterile, tissue culture-treated plate is not necessary. If the cells are going to be in the plate overnight or longer a sterile, tissue culture-treated plate should be used, and aseptic techniques should be followed.
The need for tissue culture-treated or coated plates depends on the specific cell lines used, and how the cells are going to be treated in the course of the assay. Cells can be broadly divided into three classes of cells:
- Strongly adherent cells
- Poorly adherent cells
- Suspension or non-adherent cells
Our sterile microplates are all tissue culture-treated to promote cell attachment and growth. The tissue culture treatment process involves exposing a polystyrene microplate to a plasma gas in order to modify the hydrophobic plastic surface to make it more hydrophilic. The resulting surface carries a net negative charge due to the presence of oxygen-containing functional groups such as hydroxyl and carboxyl. Strongly adherent cells will usually attach satisfactorily to tissue culture-treated plates. Cell lines that attach less strongly may require a plate with a coating such as poly-D-lysine or collagen which promotes attachment better than just tissue culture treatment. Cell-based assays using suspension cells are generally performed in standard tissue culture-treated plates. Coated plates are not typically used with suspension cells.
In addition to the specific cell line being used in the assay, the assay protocol itself is important in deciding the type of plate to use. For example, assays using adherent cells may include culture medium changes or wash steps in the protocol. In such cases it may be advisable to use a coated plate for the assay in order to prevent the cells from becoming detached from the plate during the assay.
Clear bottom vs. opaque plates
Microplates with clear bottoms can be useful for cell-based assays as they allow the microscopic visualization of the cells to monitor confluency, morphology and other parameters that may affect the cellular response in the assay. In addition, assays that are configured for bottom reading require clear bottom plates.
Clear bottom plates can be converted to functionally opaque plates by application of BackSeal™ Adhesive Bottom Seal. BackSeal plate seals are available in either white or black (catalog number 6005199 for white, catalog number 6005189 for black). The color of the BackSeal plate seal should match the color of the sides of the plate wells.
CulturPlate™ microplates
All CulturPlate microplates are sterile and tissue culture-treated for use with adherent or suspension cells. CulturPlate microplates are one solid color (either white or black), with no transparency. Because of this, these plates must be read using -reading plate readers (plate reader detector is located above the plate, as opposed to below the plate).
ViewPlate™ microplates
Not all ViewPlate microplates are sterile/tissue culture-treated; look for ViewPlate products that are designated as "sterile, TC-treated" for cell-based assays using adherent or suspension cells. ViewPlate microplates have a clear-bottom base, with the sides of each well a solid white or black in color. The clear-bottom base of these plates is specifically designed for microscopic visualization, which can be helpful when microscopic observation is required to check cell density and morphology. The clear-bottom base of the plate also allows for bottom-read measurements (i.e., when the plate detector is located below the plate within the plate reader). It is always possible to convert a ViewPlate microplate into a completely solid-color plate to facilitate -reading measurements (i.e., detector is located above the plate within the instrument) by using BackSeal™ plate seal. BackSeal plate seal is offered in either black or white and is an adhesive sticker-like seal that is affixed to the underside of the plate. ViewPlate microplates are also offered with poly-D-lysine or collagen coatings to help facilitate the binding of poorlyadherent cells to the surface of the plate.
ProxiPlate™ Plus TC microplates
ProxiPlate Plus TC plates are sterile, tissue-culture treated, shallow-well plates designed for low-volume assays. The bottom of the wells is raised to position the surface of the liquid in each well as close to the -reading instrument detector as possible, resulting in high signal. These plates are offered in black or white. They are solid (opaque) in color with no transparency, and therefore require measurement in -reading microplate readers.
Selection table for cell-based luminescent assays
| Plate | Description |
|---|---|
| CulturPlate |
|
| ViewPlate |
|
| VisiPlate |
|
| ProxiPlate TC |
|
Sterile, tissue-culture treated Clear-bottom, TC-treated IsoPlate™ microplates can also be used for cell-based luminescent assays. Clear-bottom IsoPlates are similar to ViewPlates in that the bottom of the plate is clear, while the sides of each well are either black or white. This makes the IsoPlate microplate suitable for bottom-reading instruments. However, there are a few differences between IsoPlates and ViewPlates. IsoPlates are manufactured by first molding 96 clear wells at a time, then molding a black or white frame around the clear wells. This makes the white- or black-colored well extend to the same depth as the clear well base and can help reduce crosstalk in bottom-reading assays. IsoPlates were originally developed for coincidence counting in a MicroBeta™ radiometric detection instrument (reading from and bottom coincidentally). While they have clear bottoms, IsoPlates are not ideal for confocal imaging (microscopic observations) because the optical clarity of the bottom is not as good as clarity is with CellCarrier™ Ultra or ViewPlate microplates. Additionally, IsoPlates are only available in a 96-well format.
Tips and FAQS
- BackSeal plate seal can be used to convert a clear-bottom plate into an opaque (solid-colored) plate for reads. BackSeal plate seal is offered in both white and black (catalog number 6005199 for white, catalog number 6005189 for black). The color of the BackSeal plate seal should be chosen to match the color of the sides of the wells of the plate.
Q. What kind of lid can I use for my plates?
A. TC-treated CulturPlates, ViewPlates, and ProxiPlates, as well as all TC-treated microplates from Revvity, are supplied with a lid. The exception to this is when the TC-culture treated plate is purchased in a case of 200. In this instance, lids are not supplied with the microplates and much be purchased separately. For 96-well plates, the catalog number for the lid is 6005619, and for 384-well and 1536-well plates the catalog number for the lid is 6007619. Lids for 96-well plates have condensation rings that align with the underlying wells. These lids will leave a small space between the lid and the well. This is necessary so cells can ‘breathe’ when growing. Lids should be removed prior to reading.
Q. Can I use a seal on my plates, or will that kill my cells?
A. If cell viability is no longer an issue, Seal-A™ adhesive plate seal (catalog number 6050185) can be used to prevent evaporation during incubation steps or during a luminescent plate read. If cell viability is an issue at the time a seal is needed, we recommend using sterile plate lids (if sterile practices need to be maintained) or breathable plate seal (if antibiotics/antifungals can be added to the culture media to prevent contamination). Breathable plate seal is available from various suppliers, including Nunc® and Corning®.
Microplates for biochemical luminescent assays
Microplates for standard, in vitro luminescent assays that do not require anchoring of cells or other reagents to the surface of the plate.
OptiPlate™ microplates
OptiPlate microplates are standard, highly-versatile microplates. These plates are offered in black or white. They are solid (opaque) in color with no transparency, and therefore require measurement in -reading microplate readers. OptiPlate microplates are available in 24-, 96-, 384 and 1536-well formats and also as a HB (high bind) version.
ProxiPlate™ microplates
ProxiPlate microplates are shallow-well plates designed for low-volume assays. The bottom of the wells is raised to position the surface of the liquid in each well as close to the -reading instrument detector as possible, resulting in high signal. These plates are offered in black or white. They are solid (opaque) in color with no transparency, and therefore require measurement in -reading microplate readers. ProxiPlate microplates are available in 96-well and 384-well formats.
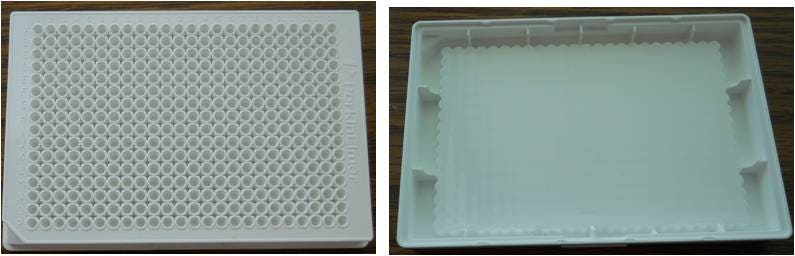
Figure 3: view of a 384-well white ProxiPlate microplate (left) and view from underneath the same plate (right). The bottom of the wells is pushed towards the surface of the plate to help increase signal while allowing use of low assay volumes.
½ AreaPlate™ microplates
"Half-AreaPlate" microplates are special plates that are designed to facilitate pipetting of low assay volumes in 96-well format. These plates have wells that are spaced to mimick the spacing of standard 96-well plates, but the actual area of each well is reduced to permit use of lower assay volumes than would be used in a typical 96-well plate. For example, a typical assay volume for a 96-well OptiPlate microplate might be 100-200 μL. A typical assay volume for a 96-well ½ AreaPlate microplate is 40-50 μL. These plates are solid (opaque) in color, offered in black or white, and require measurement in -reading microplate readers.

Figure 4: 96-well OptiPlate microplate (left) vs. 96-well ½ AreaPlate microplate (right)
AlphaPlate™ microplates
AlphaPlate microplates are light gray plates designed to reduce cross-talk while maintaining high signal in luminescent assays run in 384-well and 1536-well format. These plate provide higher signal-to-background and increased sensitivity compared to white or black plates in luminescent assays, with cross-talk similar to black plates. These plates are recommended for Alpha assays including AlphaLISA™, AlphaLISA™ SureFire® Ultra, and AlphaScreen™, as well as other biochemical luminescent assays.
ViewPlate® microplates (untreated)
ViewPlate microplates have a clear-bottom base, with the sides of each well a solid white or black in color. The clear-bottom base of ViewPlate microplates is specifically designed for microscopic visualization when needed. The clear-bottom base of the plate allows for bottom-read measurements (i.e., when the plate detector is located below the plate within the plate reader). It is always possible to convert a ViewPlate microplate into a completely solid-color plate to facilitate -reading measurements (i.e., detector is located above the plate within the instrument) by using BackSeal plate seal. BackSeal plate seal is offered in either black or white, and is an adhesive sticker-like seal that is affixed to the underside of the plate.
IsoPlate™ microplates (untreated)
Clear-well IsoPlate microplates are similar to ViewPlate microplates in that the bottom of the plate is clear, while the sides of each well are either black or white. This makes clear-well IsoPlate microplates suitable for bottom-reading instruments. However, there are a few differences between IsoPlate and ViewPlate microplates. IsoPlate microplates are manufactured by first molding 96 clear wells at a time, then molding a black or white frame around the clear wells. This makes the white- or black-colored well extend to the same depth as the clear well base, and can help reduce cross-talk in bottom-reading assays. Clear-well IsoPlate microplates were developed for coincidence counting in a MicroBeta instrument (reading from and bottom coincidentally). IsoPlate microplates that have white well bottoms but black well side walls are also offered to help minimize phosphorescence and cross-talk while maximizing signal. IsoPlate microplates are only available in 96-well format.
VisiPlate™ microplates (non-TC treated)
VisiPlate microplates have a clear-bottom base, with the sides of each well a solid white or black in color. The clear-bottom base of the plate allows for bottom-read measurements (i.e., when the plate detector is located below the plate within the plate reader). It is always possible to convert a VisiPlate microplate into a completely solid-color plate to facilitate -reading measurements (i.e., detector is located above the plate within the instrument) by using BackSeal plate seal. BackSeal plate seal is offered in both black and white, and is an adhesive sticker-like seal that is affixed to the underside of the plate. VisiPlate microplates are similar to ViewPlate and IsoPlate microplates, but are the only clear-bottom, solid-colored well plates that are offered in 24-well format. VisiPlate microplates are only offered in 24-well format.
| Plate | Description |
|---|---|
| OptiPlate |
|
| ProxiPlate |
|
| 1/2 Area |
|
| AlphaPlate |
|
| ViewPlate |
|
| IsoPlate |
|
| VisiPlate |
|
Clear-bottom IsoPlate microplates are similar to ViewPlate microplates in that the bottom of the plate is clear, while the sides of each well are either black or white. This makes the IsoPlate microplate suitable for bottom-reading instruments. However, there are a few differences between IsoPlate and ViewPlate microplates. IsoPlates are manufactured by first molding 96 clear wells at a time, then molding a black or white frame around the clear wells. This makes the white- or black-colored well extend to the same depth as the clear well base and can help reduce cross-talk in bottom-reading assays. IsoPlates were originally developed for coincidence counting in a MicroBeta® radiometric detection instrument (reading from and bottom coincidentally) Additionally, Isoplates are only available in a 96-well formation while ViewPlates are available in 96-, 384-, and 1536-well formats.

Figure 5: Photo from the bottom of a 96-well ViewPlate microplate (left) and a 96-well clear-bottom IsoPlate microplate (right). The ViewPlate microplate has a one-piece rectangular clear plastic base on the underside of the plate. The IsoPlate microplate has individual circular clear plastic well bottoms that are planar with the bottom of the white- or black-framed wells.
Tips and FAQS
- BackSeal plate seal can be used to convert a clear-bottom plate into an opaque (solid-colored) plate for reads. BackSeal plate seal is offered in both white and black (catalog number 6005199 for white, catalog number 6005189 for black). The color of the BackSeal plate seal chosen should match the color of the sides of the wells of the plate.
Q. What kind of plate seal can I use for my plates?
A. Seal-A adhesive plate seal (catalog number 6050185) can be used to prevent evaporation during incubation steps or during a luminescent plate read.
Q. What kind of lids can I use for my plates?
A. Clear, sterile lids to fit our microplates can be ordered separately For 96-well plates, the catalog number for the lid is 6005619, and for 384-well and 1536-well plates the catalog number for the lid is 6007619. Lids for 96-well plates have condensation rings that align with the underlying wells. These lids will leave a small space between the lid and the well. This is necessary so cells can ‘breathe’ when growing. Lids should be removed prior to reading.
Microplates for coated-plate assays (including chemiluminescent ELISAs)
Sometimes referred to as "solid phase assays", coated-plate assays require the anchoring of one of the assay components (protein, antibody, sample, etc.) to the surface of the microplate. Coated-plate assays use wash steps to separate bound (associating) and unbound (non-associating) reagents from the well of the plate.
OptiPlate™ HB (high-binding) plates
High-bind OptiPlate microplates are uncoated plates that are specially treated to allow passive, direct coating of antibodies, proteins, samples, and other biomolecules using standard plate coating procedures. These plates are offered in black or white. They are solid (opaque) in color with no transparency, and therefore require measurement in -reading microplate readers.
IsoPlate™ HB (high-binding) plates
High-bind IsoPlate microplates are uncoated plates that are specially treated to allow passive, direct coating of antibodies, proteins, samples, and other biomolecules using standard plate coating procedures. The bottom of the wells is clear, while the sides of each well are either black or white in color. The clear-bottom base of the plate allows for bottom-read measurements (i.e., when the plate detector is located below the plate within the plate reader). It is always possible to convert an IsoPlate microplate into a completely solid-color plate to facilitate -reading measurements (i.e., detector is located above the plate within the instrument) by using BackSeal plate seal. BackSeal plate seal is offered in either black or white and is an adhesive sticker-like seal that is affixed to the underside of the plate.
Selection table for coated-plate luminescent assays
| Plate | Description |
|---|---|
| OptiPlate HB |
|
| IsoPlate HB |
|
| 1/2 Area HB |
|
Tips and FAQS
- BackSeal plate seal can be used to convert a clear-bottom plate into an opaque (solid-colored) plate for reads. BackSeal plate seal is offered in both white and black (catalog number 6005199 for white, catalog number 6005189 for black). The color of the BackSeal plate seal should match the color of the sides of the wells of the plate.
Q. What kind of plate seal can I use for my plates?
A. Seal-A adhesive plate seal (catalog number 6050185) can be used to prevent evaporation during incubation steps or during a luminescent plate read.
Q. What kind of lids can I use for my plates?
A. Clear, sterile lids to fit our microplates can be ordered separately For 96-well plates, the catalog number for the lid is 6005619, and for 384-well and 1536-well plates the catalog number for the lid is 6007619. Lids for 96-well plates have condensation rings that align with the underlying wells. These lids will leave a small space between the lid and the well. This is necessary so cells can ‘breathe’ when growing. Lids should be removed prior to reading.
Q. Do you have any suggested plate coating protocols that I can use to bind my antibody/sample to the plate?
A. High-bind plates can be coated using any standard plate-coating method. Plates can be coated passively using the basic outline below:
- Antibody, protein, or sample (concentration of ~10 µg/mL or higher) is incubated in the plate overnight in a carbonate buffer at an appropriate temperature (room temperature or 4 degrees Celsius). Select a temperature that will help maintain stability of the antibody, protein, or sample being coated.
- Plate is washed three times with buffer (for example, 1X PBS).
- Plate is "blocked" overnight to cover the well surface area that remains (typically using BSA, sugars such as trehalose or casein, serum, etc.)
- Final washes are performed with buffer before using the plate in an assay.
For more information on plate coating, blocking, and storage, we recommend this reference:
Brown, M. C. (2011) Microtiter Plate Elisa, in Immunoassays in Agricultural Biotechnology (ed G. Shan), John Wiley & Sons, Inc., Hoboken, NJ, USA. doi: 10.1002/9780470909935.ch4
Crosstalk Studies
ATPlite
Luminescence assays are usually measured in white microplates, since the white color reflects the light to give a maximal signal, whereas black microplates absorb some of the light and give a lower signal. In comparison to other assay technologies, luminescence assays are capable of generating relatively high signal levels, so well-to-well cross-talk may potentially be an issue.
In order to compare the signal levels and cross-talk generated using different colors of microplates, an ATPlite 1-step assay was performed using a high concentration of ATP. We compared white, black and light gray plates in 96- to 1536-well plate densities. In 96-well format we also looked at black & white IsoPlates, which are microplates that have white wells within a black frame.
Cell-based luminescence assays can be run in either clear-bottom or opaque plates. We measured the signal and cross-talk in clear bottom white and black ViewPlate microplates.
In this study the cross-talk reported is the average amount measured in the four wells adjacent to the well containing the ATPlite signal. The formula used to calculate the cross-talk and platemap used in the calculation are shown below.
% Cross-talk = (Average cross-talk signal – Average blank)/(ATPlite signal – Average blank)*100
| Blank | Crosstalk Signal | Blank | ||
| Crosstalk signal | ATPlite signal | Crosstalk signal | ||
| Blank | Crosstalk signal | Blank |
Figure 6. Platemap for calculating luminescence cross-talk Alpha Technology
Materials and Methods
| Product Name | Revvity Product Number |
|---|---|
| OptiPlate-384 microplate, white | 6007290 |
| AlphaPlate-384 microplate, light gray | 6005350 |
| OptiPlate-384 F microplate, black | 6007570 |
| OptiPlate-1536 microplate, white | 6004290 |
| AlphaPlate-1536 microplate, light gray | 6004350 |
| OptiPlate-1536 F microplate, black | 6004270 |
| ProxiPlate-384 Plus microplate, white, shallow well | 6008280 |
| AlphaPlate-384 microplate, light gray, shallow well | 6008350 |
| ProxiPlate-384 Plus F microplate, black, shallow well | 6008260 |
| ViewPlate-384 microplate, white | 6007490 |
| ViewPlate-384 F microplate, black | 6007470 |
| ViewPlate-1536 microplate, white | 6004490 |
| ViewPlate-1536 F microplate, black | 6004470 |
| Black & White Isoplate-96 microplate | 6005030 |
| ATPlite 1-step Assay | 6016731 |
Equal volumes of 1 micromolar ATP dissolved in PBS and ATPlite 1-step reagent were added to the microplate and mixed for 5 minutes using a plate shaker. The total assay volumes used for the different plate formats were:
1536-well – 5 µL
384-well standard – 50 µL
384-shallow well – 10 µL
96-well – 200 µL
The volumes used are representative of typical assay volumes for these plate types. The plates were dark-adapted for 10 minutes and then read on the EnVision® multimode plate reader using ultrasensitive luminescence detection with a 0.1 second read time for opaque plates and 384-well ViewPlate microplates. 1536-well ViewPlate microplates were read using standard luminescence detection.
Results
The % cross-talk calculated for each plate density is shown below.
1536-Well
| 1536-well white OptiPlate microplate | 1536-well AlphaPlate microplate | 1536-well black OptiPlate microplate | 1536-well white ViewPlate microplate | 1536-well black ViewPlate microplate | |
|---|---|---|---|---|---|
| ATPlite signal | 1,247,280 | 969,160 | 29,520 | 118,378 | 7476 |
| Cross-talk signal | 25,030 | 4,110 | 20 | 4,648 | 123 |
| Blank | 200 | 40 | 13 | 150 | 20 |
| % Cross-talk | 1.99 | 0.42 | 0.02 | 3.80 | 1.38 |
384-Shallow well
| 384-well white ProxiPlate microplate | 384-shallow well AlphaPlate microplate | 384-well black ProxiPlate microplate | |
|---|---|---|---|
| ATPlite signal | 3,814,920 | 2,044,240 | 212,720 |
| Cross-talk signal | 4,510 | 40 | 10 |
| Blank | 67 | 13 | 33 |
| % Cross-talk | 0.12 | 0.001 | 0.01 |
384-Standard well
| 384-well white OptiPlate microplate | 384-well AlphaPlate microplate | 384-well black OptiPlate microplate | 384-well white ViewPlate microplate | 384-well black ViewPlate microplate | |
|---|---|---|---|---|---|
| ATPlite signal | 15,831,720 | 8,761,080 | 411,400 | 1,608,680 | 60,000 |
| Cross-talk signal | 76,590 | 1,310 | 9 | 7,900 | 50 |
| Blank | 333 | 240 | 13 | 205 | 7 |
| % Cross-talk | 0.48 | 0.01 | 0.00 | 0.48 | 0.07 |
96-well
| 96-well white OptiPlate microplate | 96-well IsoPlate microplate | 96-well black OptiPlate microplate | |
|---|---|---|---|
| ATPlite signal | 22,701,360 | 25,462,320 | 1,007,520 |
| Cross-talk signal | 3,060 | 140 | 10 |
| Blank | 80 | 127 | 9 |
| % Cross-talk | 0.01 | 0.00 | 0.00 |
Summary
White plates give higher signal levels than black plates, but this is also accompanied by higher well-to-well cross-talk. The amount of cross-talk increased as the plate well-density increases. Cross-talk is highest in 1536-well plates, and less so in 384-well plates.
Cross-talk is not significant in either 384-shallow well or 96-well plates. These two types of plates are constructed so that the wells are connected by only a narrow piece of plastic.
If cross-talk is an issue using a white plate, switching to a gray plate may be preferable to using a black plate, since cross-talk is significantly reduced without a major loss in total signal.
Alpha Technology
White microplates rather than black plates are usually recommended for luminescence assays, since the white color reflects the light signal, resulting in a higher total assay signal. However, at high signal levels or high plate well-densities, i.e. 1536-well plates, some of the light may be transmitted through the walls of a well and be detected in an adjacent well. Switching from a white plate to a black plate can reduce the amount of cross-talk, but at the same time the overall assay signal will be significantly reduced. As an alternative to using black plates to reduce well-to-well cross-talk, Revvity has developed light gray AlphaPlate™ microplates. The advantage of AlphaPlate microplates compared to black plates is that cross-talk can be reduced with much less reduction in to total assay signal.
We have conducted a study comparing the performance of AlphaScreen assays in white OptiPlate microplates to AlphaPlate microplates in various plate well-densities. AlphaScreen Omnibeads™ were used to generate the signal that was detected using the EnVision plate reader. The total signal and the well-to-well crosstalk were compared for the two plate types. In addition, we also compared the Z’-factor in white OptiPlate vs. AlphaPlate microplates.
Alpha assays such as AlphaLISA™, AlphaLISA ™ SureFire® Ultra™, and AlphaScreen ™ produce a luminescent output signal when Donor beads that are excited at 680 nm transfer energy mediated by singlet oxygen to Acceptor beads in close proximity. When singlet oxygen comes in contact with Acceptor beads, an energy-transfer cascade is initiated that culminates in light output at 520-620 nm for AlphaScreen and 615 nm for AlphaLISA assays. When using Revvity’s EnVision™ or EnSpire™ multilabel readers the excitation energy source is a high power laser. The power level of this laser is considerably higher than that of a standard flash lamp used as an excitation source for other detection technologies.
Materials and Methods
| Product Name | Revvity Product Number |
|---|---|
| OptiPlate-384 microplate, white | 6007290 |
| AlphaPlate-384 microplate, light gray | 6005350 |
| OptiPlate-1536 microplate, white opaque | 6004290 |
| AlphaPlate-1536 microplate, light gray | 6004350 |
| ProxiPlate-384 Plus microplate, white, shallow well | 6008280 |
| AlphaPlate-384 microplate, light gray, shallow well | 6008350 |
| ½ AreaPlate-96 microplate, white | 6005560 |
| AlphaScreen Omnibeads | 6760626M |
A solution of Omnibeads was prepared by adding 100 µL of Omnibeads to 25 mL of PBS. The Omnibead solution was added to the high signal well and PBS to the other wells of a white OptiPlate microplate and the corresponding AlphaPlate microplate. The volumes used for the different plate formats were:
- 1536-well – 5 µL
- 384-well standard – 20 µL
- 384-shallow well – 10 µL
- 96-well ½ AreaPlate microplate – 50 µL
The volumes used are representative of typical assay volumes for these plate types. The plates were read on the EnVision® multimode plate reader using the standard factory-defined AlphaScreen settings.
An example of the AlphaScreen cross-talk pattern that is typically seen is shown in Figure 7. The well in the center of the grid was filled with a solution of Omnibeads, and all other wells with PBS. This specific data was generated in a 1536-well OptiPlate microplate; however, the same general pattern is seen in other plate well-densities. The two adjacent wells in the same row as the well containing OmniBeads show the greatest amount of cross-talk; lower cross-talk is also seen in adjacent wells in the rows above and below.

Figure 7. AlphaScreen cross-talk pattern measured in 1536-well white OptiPlate microplates.
In the results section below the cross-talk reported is the amount in the two adjacent wells in the same row as the well containing Omnibeads. The formula used to calculate the cross-talk is shown below, which uses the platemap defined in Figure 8.
% Cross-talk = (Average cross-talk signal – Average blank)/(High signal – Average blank)*100
| Blank | Blank | |||
| Crosstalk signal | High signal | Crosstalk signal | ||
| Blank | Blank |
Figure 8. Platemap for calculating AlphaScreen % cross-talk
The Z’-factor was determined using plates with one half of the wells filled with Omnibeads and the other half of the wells filled with PBS. OmniBeads were added at two concentrations to generate both a high total signal and a low total signal to examine the impact of the signal level on the observed Z’-factor.
Results
The tables below show the AlphaScreen crosstalk in white Optiplate microplates compared to AlphaPlate microplates in various plate well-densities. 96-well ½ AreaPlate microplates were tested for reference even though there is no corresponding ½ area AlphaPlate microplate. In all of the comparisons, there was less cross-talk in AlphaPlate microplates than in white OptiPlate microplates.
| 1536-well OptiPlate microplate | 1536-well AlphaPlate microplate | |
|---|---|---|
| High signal | 231,395 | 147,992 |
| Cross-talk signal | 3,294 | 570 |
| Blank | 12 | 4 |
| % Cross-talk | 1.4 | 0.4 |
| 384-well ProxiPlate microplate | 384-shallow well AlphaPlate microplate | |
| High signal | 352,804 | 132,388 |
| Cross-talk signal | 3,086 | 476 |
| Blank | 442 | 146 |
| % Cross-talk | 0.75 | 0.25 |
| 384-well OptiPlate microplate | 384-well AlphaPlate microplate | |
|---|---|---|
| High signal | 465,932 | 178,696 |
| Cross-talk signal | 2,944 | 258 |
| Blank | 310 | 90 |
| % Cross-talk | 0.57 | 0.01 |
| 96-well 1/2 AreaPlate microplate | |
| High signal | 386,300 |
| Cross-talk signal | 56 |
| Blank | 3 |
| % Cross-talk | 0.003 |
A potential drawback of the use of AlphaPlate microplates is that the reduction of cross-talk achieved when switching from a white to a gray plate is accompanied by a reduction in the overall signal. This narrows the signal window and can possibly decrease the Z’-factor for an assay. The magnitude of the signal reduction varies depending of the plate well-density. The signal decreases the least in 1536-well-plates, and is fairly significant in 384-well plates. The AlphaPlate microplate signals as a percent of the corresponding white OptiPlate signals for the different well densities are:
1536-well – 64%
384-shallow well – 38%
384-regular well – 38%
A test was therefore performed to determine the affect of the decreased signal window on the Z’-factor. Omnibeads were added at two concentrations to generate both a high total signal and a low total signal. The two signals were compared to a PBS blank in 1536-well and 384-shallow well OptiPlate and AlphaPlate microplates in order to determine the Z’-factor. The results are summarized in the tables below. For both the high and low total signal levels the decreased signal window when switching from white OptiPlate microplates to AlphaPlate microplates did not impact the Z’-factor.
1536-Well Plates Z’-factor
| OptiPlate High | AlphaPlate High | OptiPlate Low | AlphaPlate Low | |
|---|---|---|---|---|
| Average Signal | 266,522 | 174,772 | 39,578 | 26,304 |
| SD | 7,029 | 4,310 | 1,101 | 739 |
| % CV | 2.64 | 2.47 | 2.78 | 2.81 |
| Average Blank | 22 | 8 | 11 | 7 |
| STDev | 6 | 3 | 4 | 3 |
| Z' -factor | 0.92 | 0.93 | 0.92 | 0.92 |
384-Shallow Well Plates Z' -factor
| ProxiPlate High | AlphaPlate High | ProxiPlate Low | AlphaPlate Low | |
|---|---|---|---|---|
| Average Signal | 205,346 | 74,556 | 53,947 | 18,621 |
| StDev | 6,788 | 2,968 | 2,623 | 896 |
| % CV | 3.31 | 3.98 | 4.86 | 4.81 |
| Average Blank | 371 | 160 | 391 | 157 |
| SD | 54 | 29 | 58 | 22 |
| Z' -factor | 0.90 | 0.88 | 0.85 | 0.85 |
Conclusions
- The highest percentage of AlphaScreen cross-talk is seen in 1536-well OptiPlate microplates where it is ~1.5%
- The next highest amount of crosstalk occurs in 384-shallow well ProxiPlate microplates in which it is about half the amount observed in a 1536-well plate.
- Switching from a white OptiPlate microplate to an AlphaPlate microplate results in a significant, but not complete reduction in cross-talk.
- The total signal level obtained using AlphaPlate microplates is lower compared to OptiPlate microplates; however, Z'-factors measured in AlphaPlate microplates are comparable to those seen using OptiPlate microplates.
Plate Recommendations for Revvity Luminescence Assays
ATPlite™ and ATPlite 1step assays
We recommend white or black tissue culture-treated, sterile CulturPlate or ViewPlate microplates for ATPLite assays. If needed, dark adapt white plates for 10 minutes to reduce plate phosphorescence. Black plates show minimal plate phosphorescence and therefore there is no need for dark adaptation. If sterility is not an issue we recommend AlphaPlate microplates.
britelite™ plus, neolite™, steadylite™ plus, sensitlite™, and twinlite™ assays
For optimum light yield, low background and minimum well-to-well cross-talk, white microplates should be used. We recommend the use of a sterile, tissue culture-treated CulturPlate or ViewPlate microplates (when visual inspection of cells is preferred), or an OptiPlate microplate if sterility and TC-treatment are not necessary for a particular assay. Black plates can also be used when very high signals are expected. Black plates will reduce well-to-well cross-talk but will also quench the light output.
AequoScreen™ and PhotoScreen™ assays
If a sterile, TC-treated plate is needed for your assay, we recommend white CulturPlate microplates or white-walled, clear bottom ViewPlate TC microplates. If cross-talk is an issue with the clear-bottom ViewPlate microplates, you can try black-walled, clear-bottom ViewPlate TC microplates. If you are injecting the cells into the wells and are using the cells in suspension, and no plate sterility or TC-treatment is required, we recommend white OptiPlate microplates.
When using an MDS FLIPR™ or Hamamatsu FDSS inject and read flash luminescence imager, black clear bottom ViewPlate microplates are recommended.
Plate seals
Revvity offers a variety of plate seals. Seal™ is a range of plate seals that are applied to the surface of the plate, and are used to prevent evaporation or radioactive contamination during assay incubation steps and/or plate reading measurements. Seal-A can be left on the plate during luminescence, AlphaScreen ™ , AlphaLISA™ , AlphaLISA™ SureFire® Ultra™, and radiometric measurements. Seal plate seals have spectral properties that may interfere with other types of assay measurements (absorbance assays, colorimetric assays, fluorescence assays). For these types of assays, you should compare the plate measurement with and without a Seal plate seal to test for interference. BackSeal plate seals are plate seals that are applied to the bottom of the plate. BackSeal plate seals can be used to seal the bottom of a filter plate prior to the addition of scintillation cocktail, preventing leakage. BackSeal plate seals can also be used to change a clear-bottom plate into a white- or black-bottom plate in order to reduce cross-talk during -reading measurements.
| Product | Type of Seal | Plate Format | Number of Seals | Catalog Number |
|---|---|---|---|---|
| Seal-A | Clear adhesive seal | All | 100 | 6050185 |
| Clear adhesive seal, for 24-well plates | 24-well | 100 | 6005189 | |
| Black adhesive seal | All | 100 | 6050173 | |
| Seal-B | Adhesive seal for PCR plates | All | 100 | 6050174 |
| Seal-S | Heat seal for polystyrene plates | 96-well | 100 | 6050192 |
| BackSeal | White adhesive seal | 96-, 384- well | 55 | 6005199 |
| Black adhesive seal | 96-, 384- well | 55 | 6005189 |
Custom plate services at Revvity
Revvity offers custom microplates services, including:
- Bulk ordering
- Fast and flexible plate barcoding
- Biological plate coating including – poly-D-lysine, collagen, streptavidin, and antibody coating
- Custom treatments including – tissue-culture, high protein binding and low protein binding
- Custom sterilization
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























