Overview
Accurately determining gastric emptying rates is crucial for understanding the physiological mechanisms underlying alterations in gastric motility. In fact, a pharmacological agent’s effects on gastric emptying can determine its potential as a therapeutic. Currently, the methods used for preclinical determination of gastric emptying rates involve terminal assessment of dye or radioactive tracers within the stomach. These methods require the use of many animals, incurring high financial and time costs.
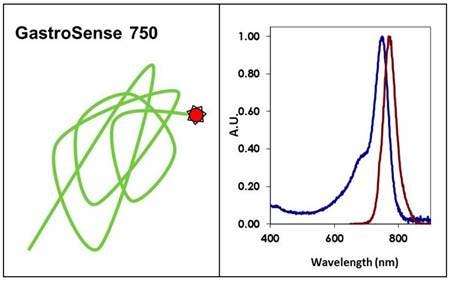
IVISense™ Gastrointestinal 750 (formerly GastroSense) is a near-infrared fluorescent imaging probe designed to monitor and quantify gastric and intestinal emptying non-invasively in murine models in vivo. It may also be used as an anatomical marker for the gastrointestinal tract.

Figure 1: IVISense Gastrointestinal 750 (formerly GastroSense) is a non-targeted physiologic near-infrared fluorescence (NIRF) in vivo imaging probe that can be delivered by oral gavage to the stomach of mice for imaging. Quantitative data regarding the rate at which the stomach empties under the influence of different diets or pharmacologic intervention can be acquired over time.
Products and catalog numbers
| Product | Catalog Number | Ex/Em wavelength (nm) | Molecular weight (g/mol) | Validated Experiments | Applications |
|---|---|---|---|---|---|
| IVISense Gastrointestinal 750 | NEV11121 | 750/770 | 40,000 | In vivo/ex vivo |
Gastric motility or gastric emptying |
Using IVISense Gastrointestinal 750 probe for in vivo/ex vivo studies
- IVISense Gastrointestinal 750 can be used for monitoring of gastric emptying and motility.
- IVISense Gastrointestinal 750 may be reconstituted with 1x PBS. A methylcellulose solution may also be usedused, or it may be reconstituted with a liquid diet supplement solution.
- The recommended procedure for in vivo imaging with IVISense Gastrointestinal 750 is administration via gavage or in solid food (egg yolk) and then imaging at the desired time points beginning immediately after ingestion.
- When administering IVISense Gastrointestinal 750 as part of a solid diet, apply the reconstituted solution to egg yolk. When added to a solid diet it may be easier to reconstitute the vial of IVISense Gastrointestinal 750 with 300 μL of 1x PBS and use 25 μL per mouse. Clearance times will depend on the method of administration and the animal model used. For example, when gavaged as a liquid in normal healthy mice the average half-life in the stomach may be as little as 15-30 minutes though times will vary significantly based on age, sex and strain of animal. When IVISense Gastrointestinal 750 is administered as part of a solid diet clearance half-lives typically increase. IVISense Gastrointestinal 750 remains localized in the GI tract. It does not appear to be absorbed into the bloodstream.
- Imaging in Gastric Emptying: IVISense Gastrointestinal 750 may be used to study the effect of drugs or disease progression on gastric emptying or gastric motility.
- Imaging as an anatomical marker: IVISense Gastrointestinal 750 may be used as an anatomical reference marker for imaging the gastrointestinal tract.
View instructions on setting up an in vivo mouse experiment with IVISense Gastrointestinal 750 fluorescent probe.
| Product | Route of Injection | Mouse Dose (25 g) | Rat Dose (250 g) | Blood t 1/2 | Tissue t 1/2 | Optimal imaging time | Optimal Re-injection Time (complete clearance) | Route of Metabolism/ background tissue | FMT and IVIS settings |
|---|---|---|---|---|---|---|---|---|---|
| IVISense Gastrointestinal 750 | oral gavage | 0.25 nmol | 0.75-2.5 nmol | N/A | N/A | Kinetic 5 min - 3 h |
3- 5 h | Stomach, intestines | FMT 750/770 IVIS 745/800 |
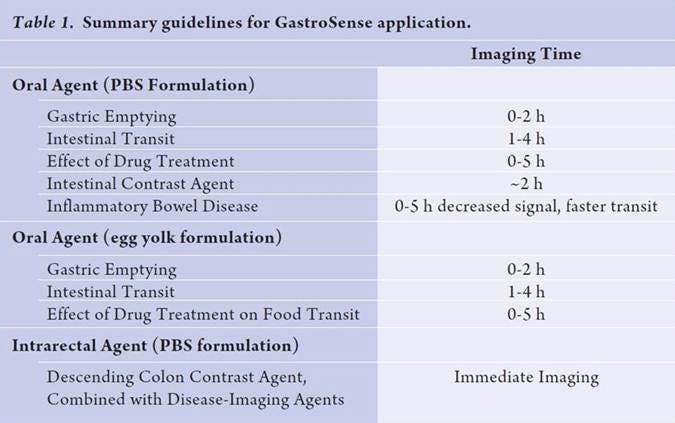
Table: IVISense Gastrointestinal 750 (formerly GastroSense) imaging times by application
In Vivo/Ex Vivo Imaging
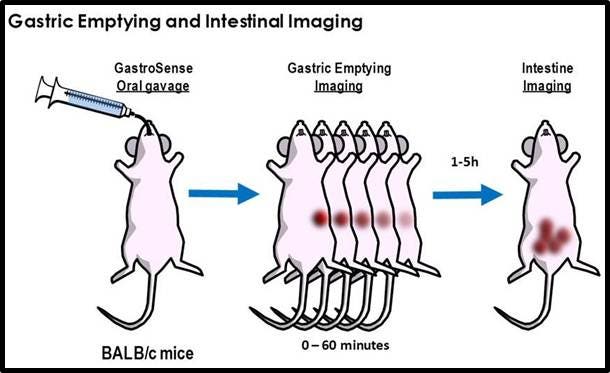
Figure 2: Gastric emptying and intestinal imaging. Mice are orally gavaged with IVISense Gastrointestinal 750 (formerly GastroSense) and gastric emptying rates can be assessed.
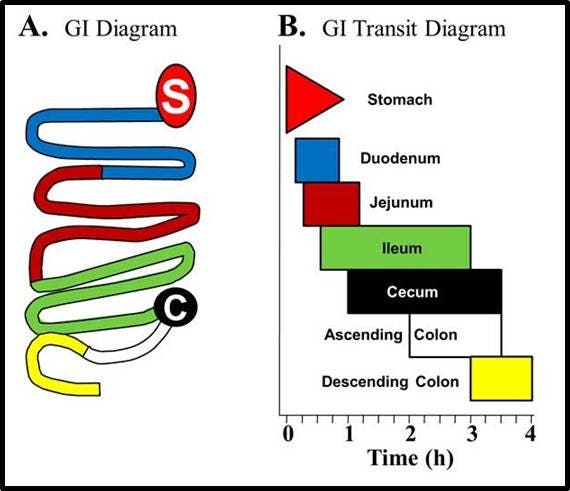
Figure 3: IVISense Gastrointestinal 750 (formerly GastroSense) was administered by oral gavage in PBS and gastrointestinal tissue was excised to determine probe localization. A) Schematic diagram of the different regions of the gastrointestinal tract. B) Diagrammatic representation of IVISense Gastrointestinal 750 localization over time based on two studies.
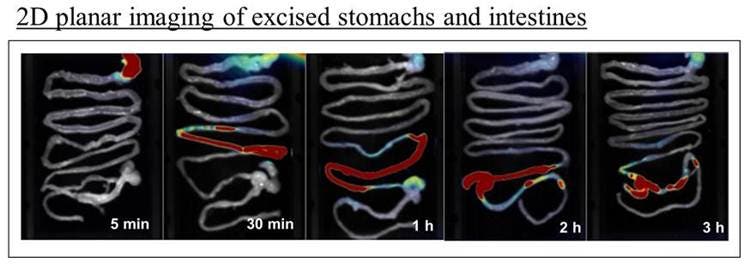
Figure 4: Ex vivo planar near-infrared images of excised gastrointestinal tissue from representative mice at five different time points.
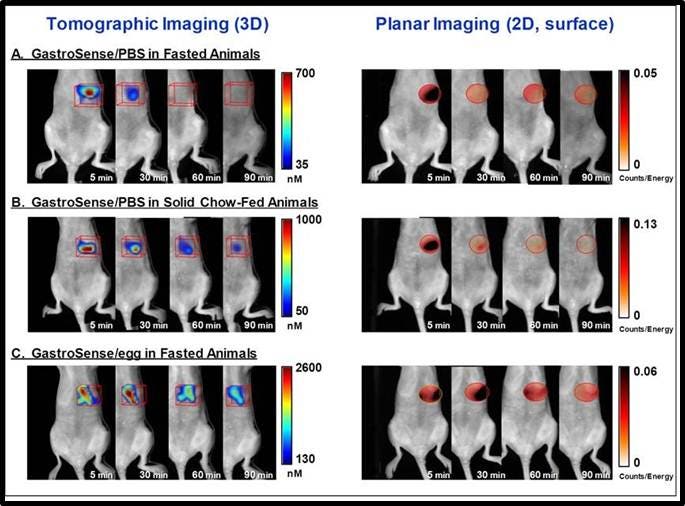
Figure 5: Imaging the stomach. IVISense Gastrointestinal 750 (formerly GastroSense) was administered by oral gavage in PBS (A, B) or fed to animals by mixing it in egg yolk as food (C). Mice were either fasted (A, C), or they were allowed free access to standard mouse chow (B). Fluorescence tomographic datasets were acquired by imaging of the gastric region over time. The left panel shows tomographic imaging, and the right panel shows surface planar imaging. Improved sensitivity of detection is achieved by deep tissue tomographic imaging, with surface imaging signal dropping off much more rapidly. IVISense Gastrointestinal 750 formulated in PBS and fed to fasted animals (A), shows rapid clearance from the stomach, whereas allowing mice access to solid chow (B) or formulation of the probe in egg yolk as a way of tracking digestion of solid food (C) clearly delays gastric emptying.
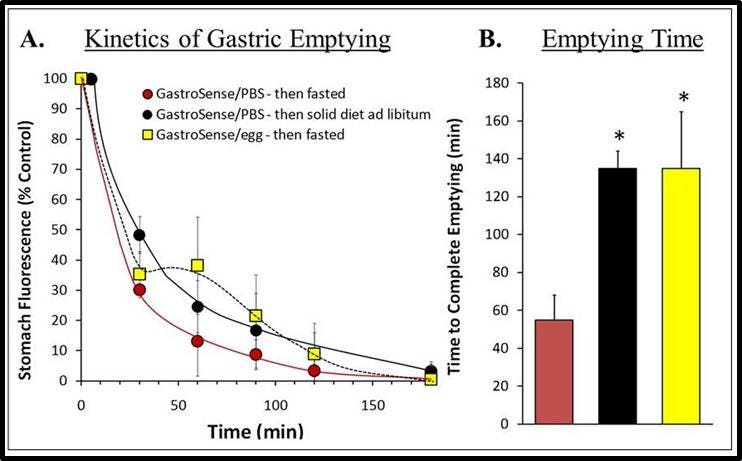
Figure 6: Quantifying Fluorescence. Fluorescence molecular tomographic imaging datasets for study groups represented by mice shown in Figure 5 were used to quantify fluorescence. A) Results in pmol were normalized to 100 % fluorescence at the starting time. B) The time to 90% gastric emptying was determined for each mouse within each group and averaged to determine complete emptying time. This analysis provided a very robust measurement of the effects of solid food on gastric motility. Asterisks indicate statistical significance ( p < 0.01).
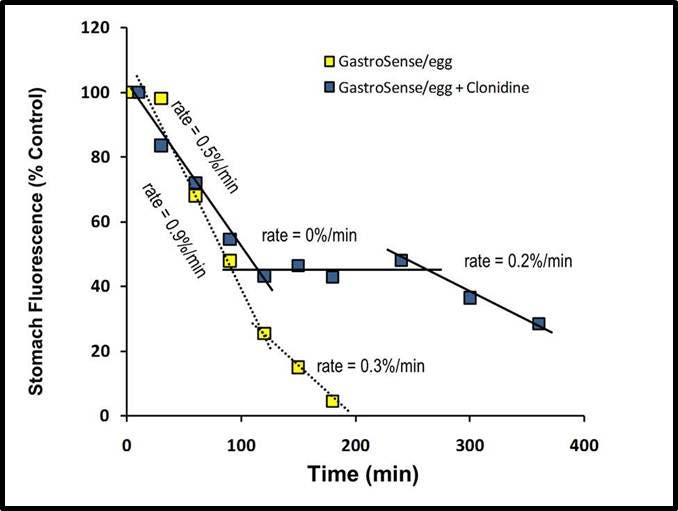
Figure 7: Inhibiting gastric motility with clonidine. Mice were treated with clonidine (1 mg/kg), a gastric emptying inhibitor, by intraperitoneal injection. Fifteen minutes after treatment with clonidine, IVISense Gastrointestinal 750 (formerly GastroSense), formulated in egg yolk, was fed to the mice. The mice were then imaged over time. Fluorescence tomographic datasets were analyzed by placing three dimensional ROIs for the stomach, cecum, and intestinal region. Intestine signal quantification was determined by subtraction of cecum fluorescence artifacts at each time point. All fluorescence was thresholded minimally at 10% of the initial fluorescence concentration.
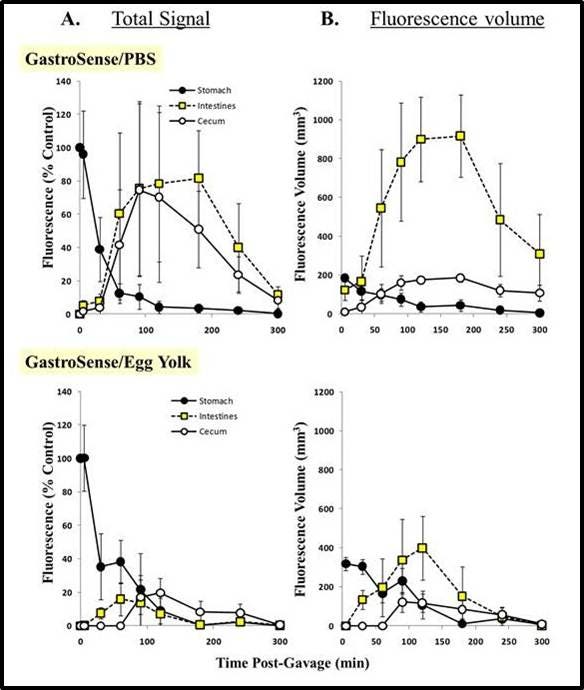
Figure 8: Imaging gastric transit. IVISense Gastrointestinal 750 (formerly GastroSense), formulated in PBS, was administered by oral gavage, and mice (n = 6) were imaged over time. Fluorescence tomographic datasets were analyzed by placing three dimensional ROIs for the stomach, cecum, and intestinal region. Intestine signal quantification was determined by subtraction of cecum fluorescence artifacts at each time point. All fluorescence was thresholded minimally at 10% of the initial fluorescence concentration.
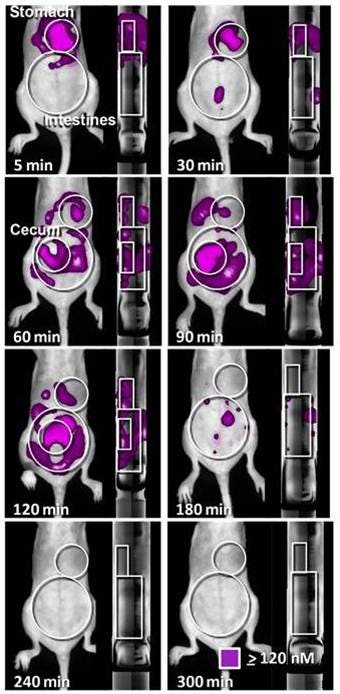
Figure 9: Fluorescence Volume Assessment. IVISense Gastrointestinal 750, formulated in egg yolk, was consumed by mice, and animals (n = 6) were imaged over time. Fluorescence datasets were represented as isosurfaces for fluorescence > 10% of the mean stomach signal ROIs for the stomach and intestinal regions. Intestine signal quantification was determined by subtraction of cecum fluorescence artifacts at each time point.
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. The information does not constitute medical advice and must not be used or interpreted as such. Consult a qualified veterinarian or researcher for specific guidance or use information. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment. Users are solely responsible for complying with all relevant laws, regulations, and institutional animal care and use committee (IACUC) guidelines in their use of the information provided.




























