Overview
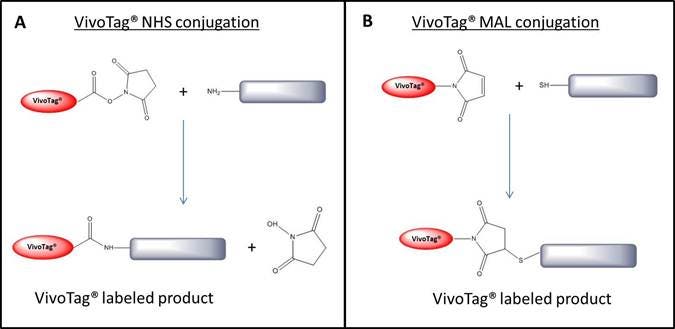
IVISense™ fluorescent dyes (formerly VivoTag™) are optimized NIR dyes for labeling molecules including peptides, small molecules, proteins, macromolecules, and nanoparticles. They are available as either NHS esters or maleimide reactive dyes which makes conjugation to either free amine (-NH3) and free thiol (-SH) containing molecules possible. IVISense fluorescent dyes have been optimized for both in vivo and in vitro research applications. Figure 1 below shows the conjugation reaction.

Figure 1: Conjugation reaction for NHS ester (A) and maleimide (B) IVISense Fluorescent Dyes (formerly VivoTag). IVISense fluorescent dye labeled conjugates have been validated in both IVIS™ and FMT™ imaging systems. In Figure 2 below, Nu/nu mice were orthotopically injected with the bioluminescent cell line MDA-MB-231-luc2. An antibody-conjugate labeled with IVISense fluorescent dye with specifity for the tumor cells was injected and compared to a IVISense fluorescent dye labeled IgG with no tumor specificity.
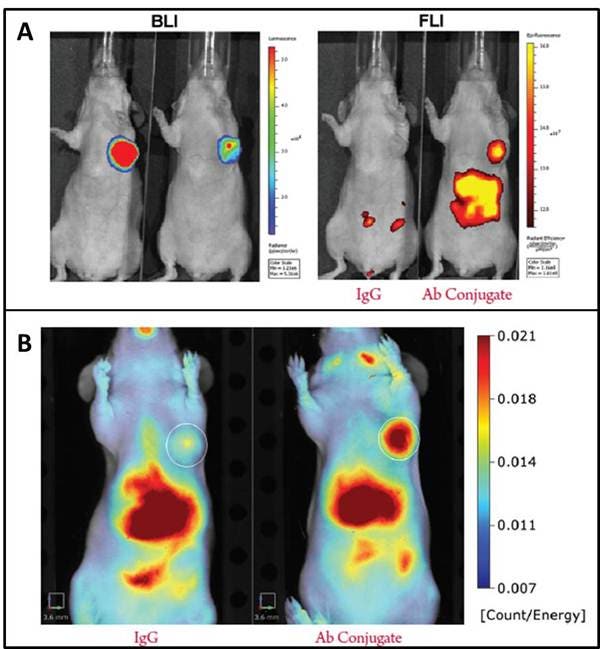
Figure 2: Imaging IVISense Fluorescent Dye conjugates on IVIS and FMT imaging systems. A. Bioluminescence (left) and fluorescence intensity (right) were measured using an IVIS spectrum. In both mice the biolumenscence from the tumor cell line is strong, but only the mouse on the right, injected with the tumor specific IVISense Fluorescent Dye conjugate shows fluorescence at the tumor site. B. FMT reflectance mode is used to show the mouse on the right, injected with the tumor specific IVISense fluorescent dye conjugate, a strong signal in the area of the of the orthotopically injected tumor. The high signal in the liver is due to the distribution kinetics of the antibody. All images shown are at 24 hours post injection of the antibody conjugate.
Products and catalog numbers
| Fluorescent Dye | Reactive with | Abs max (nm) | Em max (nm) | Epsilon (M-1cm-1) | Molecular weight* (g/mol) | Settings for in vivo imagers | Filters for microscopy and flow cytometry | Catalog numbers |
|---|---|---|---|---|---|---|---|---|
| IVISense 645 NHS Fluorescent Dye | Free amines (lysines) | 643 | 660 | ~210,000 | 1393 | FMT 635/655 IVIS 640/660 with spectral unmixing |
Microscopy: Cy 5 Flow Cytometry: 705/70 |
1 mg: NEV11173 5 mg: NEV11174 |
| IVISense 645 MAL Fluorescent Dye | Free thiols (cysteines) | 643 | 660 | ~210,000 | 1418 | FMT 635/655 IVIS 640/660 with spectral unmixing |
Microscopy: Cy 5 Flow Cytometry: 705/70 |
1 mg: NEV11273 5 mg: NEV11274 |
| IVISense 680 NHS Fluorescent Dye | Free amines (lysines) | 665 | 688 | ~210,000 | 1856 | FMT 680/700 IVIS 675/720 |
Microscopy: Cy 5.5 Flow Cytometry: 712/21 |
1 mg: NEV11119 5 mg: NEV11120 |
| IVISense 680 NHS Fluorescent Labeling Kit | Free amines (lysines) | 665 | 688 | ~210,000 | 1856 | FMT 680/700 IVIS 675/720 |
Microscopy: Cy 5.5 Flow Cytometry: 712/21 |
1 kit: NEV11118 (2 x 0.25 mg) |
| IVISense 680 MAL Fluorescent Dye | Free thiols (cysteines) | 669 | 688 | ~210,000 | 1881 | FMT 680/700 IVIS 675/720 |
Microscopy: Cy 5.5 Flow Cytometry: 712/21 |
1 mg: NEV11219 5 mg: NEV11220 |
| IVISense 680 NHS Fluorescent Self-Quenching Dye | Free amines (lysines) | 673 | 691 | ~230,000 | 1439 | FMT 680/700 IVIS 675/720 |
Microscopy: Cy 5.5 or Cy 7** Flow Cytometry: 780/60 |
1 mg: NEV10121 5 mg: NEV10122 |
| IVISense 750 NHS Fluorescent Self-Quenching Dye | Free amines (lysines) | 750 | 775 | ~240,000 | 1183 | FMT 750/770 IVIS 745/800 |
Microscopy: Cy 5.5 or Cy 7** Flow Cytometry: 780/60 |
1 mg: NEV10123 5 mg: NEV10124 |
| IVISense 750 MAL Fluorescent Self-Quenching Dye | Free thiols (cysteines) | 750 | 775 | ~240,000 | 1208 | FMT 750/770 IVIS 745/800 |
Microscopy: Cy 5.5 or Cy 7** Flow Cytometry: 780/60 |
1 mg: NEV11223 5 mg: NEV11224 |
| IVISense 800 NHS Fluorescent Dye | Free amines (lysines) | 785 | 810 | ~200,000 | 1464 | FMT 790/810 IVIS N/A |
Microscopy:N/A Flow Cytometry: N/A |
1 mg: NEV11107 5 mg: NEV11108 |
Table 1: Available IVISense Fluorescent Dyes from Revvity
*Molecular weight shown is for the TEA salt form
**For 750 nm probes, Cy 5.5 filters are recommended for microscopy for lower acquisition times.
Choosing the right IVISense Fluorescent Dye
Dye Wavelength: Be sure to choose the proper wavelength that your instrument is capable of measuring effectively. For in vitro applications such as microscopy, dyes above 750 nm can't typically be imaged. For deep tissue measurements in vivo however, longer wavelength dyes such as IVISense 750 or IVISense 800 are preferred provided your in vivo imager has the proper filter sets.
IVISense NHS vs. IVISense MAL: IVISense NHS dyes are can be used for labeling free amines (typically lysine residues), while IVISense MAL dyes are able to be used for labeling free thiols (typically cysteine residues).
IVISense Fluorecent Dyes vs. IVISense Fluorescent Self-Quenching Dyes: When a protein is labeled with multiple IVISense Fluorescent Self-Quenching Dye molecules, there is a shift in the absorbance of the dye, which will ultimately reduce the signal if using the filter sets listed above. Only choose IVISense Fluorescent Self-Quenching Dye if you are sure your labeling ratio of dye: protein will be 1:1. If multiple dyes are to be conjugated onto your protein, the non 'Self-Quenching' versions should be chosen.
Package Size: Standalone IVISense Fluorecent Dyes are available in 1 mg and 5 mg package sizes. Typically, you can label 10-20 mg of antibody using 1 mg of dye, but we do recommend trying different dye: protein conjugation ratios to determine the optimal conditions for your protein of interest.
What do I need to perform a fluorescent dye conjugation?
You will need:
- Appropriate IVISense Fluorescent Dye labeling reagent (see Table 1 in section above for catalog numbers)
- Appropriate conjugation buffer
- For NHS ester, 50 mM carbonate/biocarbonate buffer, pH 8.3-8.5
- For maleimide, appropriate buffer (e.g., 100 mM PBS, 100 mM TRIS, or 100 mM HEPES buffer, pH 6.5-7.0)
- Dimethylsulfoxide (DMSO)
- Conical collection tubes
- Appropriate filtration column
- For NHS ester, Bio-Rad Bio-Gel P-100
- For Maleimide, Sephadex G-25
Or:
- IVISense 680 NHS Fluorescent Labeling Kit which contains all of the material listed above
And:
- 0.2 µm syringe filter to filter the purified conjugate
- Absorbance reader (UV spectrophotometer, NanoDrop spectrophotometer) to determine the degree of labeling
Protocols and calculations
General overview of conjugation protocol
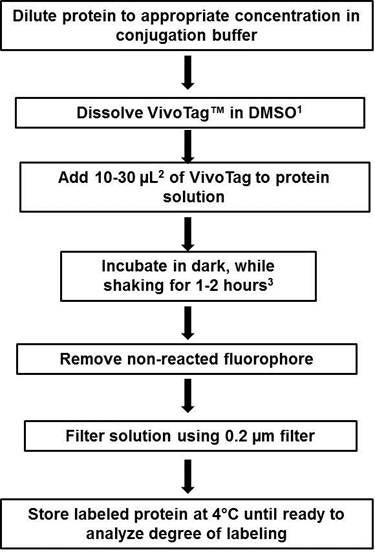
Figure 3: Flow chart of conjugation protocol. 1) Starting IVISense Fluorecent Dye (formerly VivoTag) stock solution in DMSO varies by reagent, please click on the detailed protocols below to determine the recommended stock concentration. 2) Optimal labeling ratios will vary by protein and multiple dye: protein ratios may have to be tested to determine the best condition for labeling your molecule of interest. 3) Labeling time varies by dye, please check with detailed protocols below for specific reaction times.
Please download more complete protocols for labeling proteins and antibodies with IVISense Fluorecent Dyes:
- IVISense 645 NHS Fluorescent Dye
- IVISense 680 NHS and 750 NHS Fluorescent Self-Quenching Dyes
- IVISense 680 NHS Fluorescent Dye
- IVISense 800 NHS Fluorescent Dye
- IVISense MAL Fluorescent Dyes
Determining the degree of labeling
In order to determine the degree of labeling, the absorbance of the diluted IVISense Fluorecent Dye-protein conjugate is measured at both 280 nm (to determine the amount of protein present) and at the absorbance wavelength of the IVISense Fluorecent Dye (643 nm to 790 nm, depending on the dye-See Table 1 above). There is a small amount of crosstalk at 280 nm as the IVISense Fluorecent Dyes also absorb at this wavelength, so it is necessary to subtract out this background absorbance in order to precisely calculate the degree of labeling. The amount of crosstalk varies depending on which IVISense Fluorecent Dye is chosen. Table 2 lists the amount of crosstalk for each IVISense Fluorecent Dye at 280 nm.
| IVISense Fluorescent Dye | % Crosstalk at 280 nm | Correction factor (Cf) |
|---|---|---|
| IVISense 645 NHS Fluorescent Dye | 5 | 0.05 |
| IVISense 645 MAL Fluorescent Dye | 5 | 0.05 |
| IVISense 680 NHS Fluorescent Dye | 16 | 0.16 |
| IVISense 680 MAL Fluorescent Dye | 13 | 0.13 |
| IVISense 680 NHS Fluorescent Self-Quenching Dye | 16 | 0.16 |
| IVISense 750 NHS Fluorescent Self-Quenching Dye | 6 | 0.06 |
| IVISense 750 MAL Fluorescent Self-Quenching Dye | 5 | 0.05 |
| IVISense 800 NHS Fluorescent Dye | 5 | 0.05 |
Table 2: Correction factors for IVISense Fluorecent Dyes. The correction factor is used to calculate the background absorbance of the dye at 280 nm. See sample calculation below.
Sample Calculation
Here is an example of the calculation if you labeled of an IgG labeled with IVISense 645 NHS. After purification, you dilute the conjugate 1:10 in PBS. You measure the A280 of the dilution to be 0.415 and the A643 of the dilution to be 0.786. The Epsilon (Ɛ) for IgG is 210,000 M-1cm-1 and the Epsilon (Ɛ) for IVISense 645 NHS is 210,000 M-1cm-1 (see Table 1 for all dye Epsilons).
First the A280 and the A643 need to be multiplied by the dilution factor of 10 in order to determine the stock concentration:
A280 = 0.415 x 10 = 4.15
A643 = 0.786 x 10 = 7.86
To calculate the concentration of the IgG (Pc) the correction factor needs to be multiplied by the dye absorbance and subtracted from the A280:
Pc [M] = (A280 - (A643)*Cf)/Ɛ of IgG
Pc = (4.15 - 7.86*0.05)/210,000
Pc = 4.15-0.393/210,000
Pc = 3.757/210,000 = .00001789 M or 17.89 µM IgG
To calculate the concentration of the IVISense 645 NHS:
Dc [M] = (A643)/Ɛ of IVISense 645 NHS
Dc = 7.86/210,000
Dc = 0.0000374 M or 37.4 µM IVISense 645 NHS
To calculate the degree of labeling:
Dc/Pc = 37.4 µM/17.89µM = 2.09
In this case, there are 2.09 IVISense 645 NHS molecules for every molecule of IgG. Ideal dye-to-protein labeling ratios are typically between 2 and 3. It is important to note that the above equations assume a path length of 1 cm (typical path length of a cuvette). If you are using a NanoDrop spectrophotometer to measure absorbance, the path length is only 1 mm so A280 and A643 values obtained by this method would have to multiplied by an additional factor of 10. Omitting this correction factor will not alter the dye-to-protein ratio calculated but will alter the µM of dye measured which will affect the amount of conjugate injected into your in vivo model organism.
Assay optimization for in vivo studies
Not all proteins or antibodies make good imaging agents, independent of target specificity. Some may have too long or short of a half-life in vivo or may excessively accumulate in non-target sites. It is a good idea to test specificity both in vitro and in vivo is possible, and to determine the in vivo biodistribution and pharmacokinetics of your IVISense Fluorescent Dye conjugate.
FAQs
General Product and Application FAQs
Q. What is the structure of IVISense Fluorescent Dye?
A. Unfortunately, the structure of IVISense Fluorescent Dye is proprietary and we are unable to disclose it to customers.
Q. What is the difference between IVISense Fluorescent Dye and IVISense Fluorescent Self-Quenching Dye?
A. IVISense Fluorescent Self-Quenching Dye refers to a shift in absorbance that the dye experiences when conjugated on a molecule with multiple dyes. The shift in absorbance upon conjugation is about 50-60 nm lower in wavelength than the free dye alone.
Q. For IVISense Fluorescent Self-Quenching Dye what does the shift in absorbance correspond to in terms of what I'll see on my instrument? Do I need to adjust any filter settings?
A. It depends on the instrument that is being used. If multiple tags in close proximity are added, then the new max wavelength will be 50-60 nm toward the blue range and if it is only one IVISense Fluorescent Dye the wavelength will be the one reported on the technical data sheet.
Q. Will there be an increase or decrease in signal based on the number of IVISense Fluorescent Dye molecules that bind to my protein?
A. This will depend on the protein being used and the method of detection being used as well.
Q. What is the difference between IVISense NHS and IVISense MAL?
A. The main difference between the regular dyes and the MAL dyes is that the MAL dyes are better suited for labeling thiol and cysteine containing molecules. The dye properties (abs, FL, ex. coeff, etc) are all the same.
Q. Can IVISense Fluorescent Dyes be used to label cells?
A. While this is possible, we instead recommend IVISense 680 Fluorescent Cell Labeling Dye (formerly VivoTrack™) for this application.
Q. Can I use IVISense Fluorescent Dyes in humans?
A. No. IVISense Fluorescent Dyes are intended for animal research use only, are not intended for diagnostic procedures and are not intended for human use.
Q. When optimizing my experiment in vivo, is there a control dye that can be used?
A. Yes, you can use IVISense Acute Vascular (formerly Genhance™) as a control to determine non-specific uptake as this dye has no specificity for any particular target in vivo (other than vasculature). It is the acid form of the IVISense Fluorescent Dyes and is available as in 680 nm and 750 nm versions. Alternatively, you could conjugate a non-targeting protein or antibody to IVISense Fluorescent Dyes and measure its specificity compared to your targeted molecule.
Labeling and Purification FAQs
Q. Can I reconstitute an IVISense Fluorescent Dye in an aqueous buffer?
A. Yes, you technically can if your experiment requires it, but the reaction needs to be done very quickly so that the NHS group does not hydrolyze and render the dye no longer reactive.
Q. What amount of dye is a good starting point for protein or antibody labeling?
A. Typically you can label 10-20 mg of antibody or protein with 1 mg of dye. This is a good starting point, but since all molecules are unique the amounts and ratios need to be optimized.
Q. I do not want to run my conjugate over a column to purify. Can I dialyze away the free dye instead?
A. For the NHS labeling reagents, we do not recommend dialysis as a way to remove free dye. For the maleimide dyes, dialysis can be used instead of a gel filtration column if desired.
Q.What mass should I use when analyzing my IVISense NHS Fluorescent Dye conjugates using mass spec?
A. This table contains the mass expected when using mass spec analysis:
| IVISense Fluorecent Dye | Mass (X) to be added to the MW of biomolecule |
|---|---|
| IVISense 645 NHS Fluorescent Dye | 974.2 |
| IVISense 680 NHS Fluorescent Dye | 1234.2 |
| IVISense 680 NHS Fluorescent Self-Quenching Dye | 1019.2 |
| IVISense 750 NHS Fluorescent Self-Quenching Dye | 865.2 |
| IVISense 800 NHS Fluorescent Dye | 1045.2 |
Q. Why does the table above contain values for the molecular weight that differ from the technical data sheet?
A.The IVISense Fluorescent Dye exist as triethylammonium series salts. The number of triethylammonium groups vary from bis triethylammonium to penta triethyammonium dyes. When running Mass Spec, depending on the conditions, it is possible to observe up to 5*101(MW of TEA) less than the number reported on the TD sheet. Also, upon Mass Spec analyses the dye will hydrolyze giving the MW of the acid form rather than the NHSE form of the dye.
Storage and Stability FAQs
Q.How long is the IVISense Fluorescent Dye powder good for?
A. Stability of the powder will vary for each product. Please check the technical data sheet for the most accurate information about storage temperature and shelf life.
Q. How long is the reconstituted IVISense Fluorescent Dye good for?
A. We recommend storing the DMSO reconstituted IVISense Fluorescent Dye at 4 °C protected from light. Under these conditions, we recommend using it within 1 week.
Q. How long is my IVISense Fluorescent Dye labeled protein or antibody good for?
A. When stored at 4 °C and protected from light, typically labeled protein is good for 4 weeks. If long term storage is needed, we recommend storing at -20 °C.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment. Users are solely responsible for complying with all relevant laws, regulations, and institutional animal care and use committee (IACUC) guidelines in their use of the information provided.




























