Manipulation/pipetting problem Overview
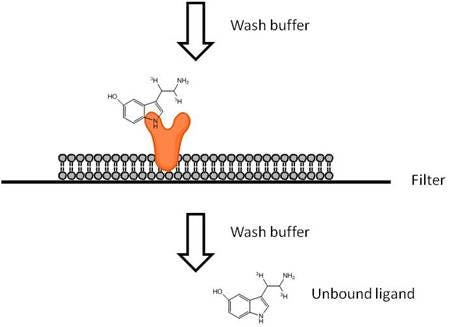
Filtration-based receptor binding studies are used to characterize receptors and to evaluate potential pharmaceutical agents by assessing their ability to interfere with the specific binding of a radiolabeled ligand to its receptor. After attaining equilibrium with receptors, excess free ligand must be separated rapidly from the bound receptor-ligand complex. This can be accomplished by filtering your receptor samples using a vacuum manifold or cell harvester. Glass fiber filters (GF/B or GF/C) are generally used. Filtration ligand binding assays are typically run using cell membranes, though whole cells may also be used.

When selecting a radioligand, there are a few factors to keep in mind:
- High specific activity: Radioligands with a high specific activity are well-suited for ligand binding assays. Specific activity indicates how much radioactivity there is per molecule of ligand and is usually given in units of Curies per millimole of ligand. Several of our 125I-labeled ligands are offered at maximum specific activity (2200 Ci/mmol if one 125I labeling site is available, 4400 Ci/mmol if two 125I labeling sites are available, etc.). This indicates that virtually every molecule of ligand provided in the stock vial is radiolabeled. For tritiated ligands (3H ligands), you should ideally pick a ligand that has a specific activity above 20 Ci/mmol. The maximum theoretical specific activity per tritium is 29 Ci/mmol (Curies per millimole of tritium). Specific activities above this value indicate that on average, each molecule of ligand has at least one tritium.
- Low non-specific binding: Hydrophobic ligands will generally show higher non-specific binding. This can sometimes be compensated by coating filters with BSA to reduce non-specific binding. Including BSA, salts or detergents in the wash or binding buffer can also help reduce non-specific binding. If the stock radiochemical is packaged in a silanized vial, this may indicate the ligand is somewhat hydrophobic
- High purity: Ideally, the ligand should have a radiochemical purity above 90%. Radiochemical purity decreases over time, and the actual rate of this degradation accelerates over time. Radiochemical purtiypurity and degradation rates for our radiochemicals can be found on each lot-specific technical data sheet.
- High selectivity: The more selective the ligand is for your receptor, the better your data will be. High selectivity indicates the ligand will mostly be recognized by only one receptor-of-interest, over other receptors that may be present in a cell membrane. Also, using membranes that over-express the receptor of interest may help reduce any potential contribution from receptors endogenously expressed in the membrane.
- Stability: If you will need to use your radioligand over an extended period of time, stability may be a factor for you. 125I-labeled ligands should generally be used within one to two months of the manufacture date. Tritiated ligands should usually be used within 3-6 months of manufacture date; however, there are exceptions to this. Degradation rates and manufacturing dates can be found on our lot-specific technical data sheets. You can also contact technical support to discuss the recommended use time for each Revvity radiochemical - our contact information is on the upper right-hand corner of this page.
- Energy: 3H releases beta energy, which can be measured on a scintillation counter after the addition of scintillant (in the form of a scintillation cocktail, Meltilex®(TM) solid scintillant, an SPA bead, etc.). The beta energy interacts with the scintillant to produce photons, which are measured by the detector. 125I releases both beta-like energy and gamma energy. If you only have access to a gamma counter, you should use a radioligand labeled with 125I. An additional factor is assay format. 125I gives off more beta-like energy than 3H. For SPA assays, either 3H or 125I-labeled ligands can be used effectively.
All Revvity cell membrane products are validated for ligand-binding in filtration assay format. Protocols can be found on each product-specific technical data sheet.
What do I need to run this assay?
If you will be using a filtermat and a vacuum manifold:
- Cell membrane expressing receptor of interest (Revvity carries receptor-transfected cell membranes)
- Radiolabeled ligand
- Cold (unlabeled) ligand as a control for non-specific binding
- Ligands and test compounds as needed
- Microplate for your binding assay (We recommend using Deep-Well plates. These plates have wells with a 1 mL volume capacity. Please check to make sure they will fit in your harvester.)
- TopSeal™-A for plate (#6051085)
- Filtermats, GF/B (#1450-521) or GF/C (#1450-421)
- Omnifilter cassette for fitting filtermat onto assay plate (make sure this is compatible with your vacuum manifold)
- Filtermat sample bag and scintillation cocktail (*see note below; we recommend Microscint™-20 (#6013621) if counting slightly damp filters, or Ultima Gold™ MV (#6013151) if you are drying your filters prior to reading)
- Optional: drying oven, set at 27-30 Celsius. Air flow is key, as you don’t want to create vapors while heating.
- Vacuum manifold or cell harvester (we recommend Filtermate Harvesters)
- High throughput radiometric detector (we recommend the MicroBeta2™ Microplate Counter
- Cassette for fitting filtermat into detector (this will depend on which instrument you are using - please contact technical support for more information)
If you will be using a UniFilter™ plate with a cell harvester:
- Cell membrane expressing receptor of interest (Revvity carries receptor-transfected cell membranes)
- Radiolabeled ligand
- Cold (unlabeled) ligand as a control for non-specific binding
- Ligands and test compounds as needed
- Microplate for your binding assay (we recommend using Deep-Well plates, Beckman #267006. These plates have wells with a 1 mL volume capacity. Please check to make sure they will fit in your harvester.)
- UniFilter plate GF/C (refer to table in next section)
- Scintillation cocktail (we recommend Microscint-20 (#6013621) if counting slightly damp plates, or Ultima Gold MV #6013151 if you are drying your plates prior to reading)
- BackSeal for plates (#6005199 - this will be used when you are going to add cocktail to the plate)
- TopSeal-A for plates (#6050185)
- Cell harvester (we recommend Filtermate™ Harvesters - make sure your harvester can accommodate a UniFilter plate)
- High throughput radiometric detector (we recommend the MicroBeta2™ counter)
- Optional: drying oven, set at 27-30 Celsius. Air flow is key, as you don’t want to create vapors while heating.
*You can substitute Meltilex™ (a solid scintillant that will melt onto the filtermat) and hot plate for melting Meltilex onto the filtermat if you prefer
*If you have the correct cassette, you could also substitute this with scintillation cocktail, BackSeal and TopSeal
Filter Plate Products and Catalog Numbers
| Plate type | Filter type | Well format | Plates | Catalog number |
| UniFilter | GF/C | 96-well | 50 | 6055690 |
| PEI-coated UniFilter | GF/C | 96-well | 50 | 6055090 |
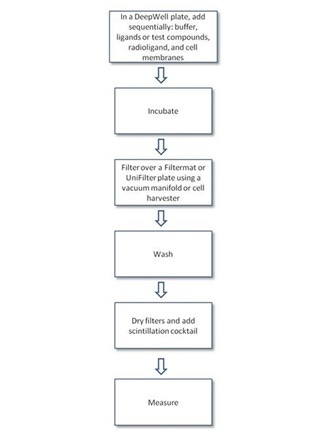
Protocol-in-brief
Detailed protocols
Coating filter plates with polyethylenimine
Treating filter plates with polyethylenimine (PEI) is a common practice to minimize ligand binding to filters. PEI is a cationic polymer that can neutralize the negative charge of the glass fiber filter. To pre-coat with PEI:
- Presoak 30 to 60 min in 0.1% to 0.5% PEI (in water) at 4°C
- Filter away PEI, then wash with ice-cold buffer prior to filtration of receptor sample
- Pretreatment with carrier proteins, serum, or detergents has also been used to minimize binding of ligands to filter plates. Pre-coating with BSA can help reduce binding of hydrophobic ligands and compounds to plastic surfaces.
- We have used PEI from Sigma-Aldrich, Cat. No. 40,870-0
Saturation assay
If you are using a Revvity cell membrane, detailed protocols for filtration ligand-binding assays are found on the cell membrane product certificate of analysis.
- Sample protocol for a saturation assay using Revvity Cat. no. RBHD47M400UA Dopamine D47 receptor and 3H-Methylspiperone
Assay optimizations
- Amount of membrane per well
- Assay volume
- Binding buffer optimization: buffer is a very important variant in every binding assay. Salt concentration, concentration of carrier proteins as well as buffering capacity and buffer type are carefully optimized. Assay validation should be performed with known ligands when new buffer is selected.
- Incubation time and temperature: binding equilibrium is temperature- and time-dependent
- Wash buffer optimization/number of wash steps
- Signal window and Z’ factor (if applicable)
- Evaluation of standard compound concentration response curves
Guides and other resources
- If you are using a Revvity cell membrane, detailed protocols for filtration ligand-binding assays are found on the cell membrane product certificate of analysis.
- NIH assay guidance website - contains information on how to develop a filter-based and SPA-based ligand-binding assay
- IUPHAR database - contains receptor-specific information on ligand affinities, lists of ligands that work with various receptors, and other information
Tips and FAQs
- It is important that the concentration of the ligand be determined by measuring a small sample of each dilution and converting the DPM to molar concentration. The accurate determination of each dilution will lead to more accurate EC50 and IC50 results.
- Most of our membrane preparations are optimized for ligand binding using GF/C filters.
- Radioligands may bind nonspecifically to components of the assay system such as tubes, pipette tips, assay plates or filters. This may lead to ligand depletion if certain binding assumptions may not be met. To test for nonspecific binding, perform an experiment in the absence of membranes. The amount of activity added can be tracked at each step of the assay to determine where any losses or nonspecific binding is occurring.
- If filters are not completely dry prior to the addition of liquid scintillant, the residual water present in the filters can interact with the scintillant to reduce counting efficiency. Dry filters require less liquid scintillant to achieve maximum signal than wetted filters. Drying filters completely may not be practical for medium or high throughput screening applications. In such cases, a cocktail compatible with wet filters should be used.
- The use of siliconized tubes and pipet tips can prevent ligand depletion (by preventing ligand adsorption to plastic).
Troubleshooting
| Problem | Cause | Possible Solution |
|---|---|---|
| High Non-specific binding | Bad membrane preparation | Try a new membrane preparation |
| Degraded radioligand/cold ligand | Use a fresh batch of radioligand or a freshly reconstituted ligand | |
| Sticky ligand | Add a carrier molecule in buffer (BSA or gelatin) | |
| Filters | Presoaking in the binding buffer with carrier moleculemolecules is important. You may also need to coat your filter with PEI | |
| Pump/vacuum issue | Check your equipment | |
| Insufficient wash | Adjust your wash buffer composition and volumes | |
| No specific binding | Bad membrane preparation | Try a new membrane preparation |
| Degraded cold ligand | Try a new lot of cold ligand | |
| Sticky ligand | Add a carrier molecule in buffer (BSA or gelatin) | |
| Pump/vacuum issue | Check your equipment | |
| Wrong filter buffer or wash buffer | Check your buffer compositions and make sure these have been optimized for your assay | |
| Low counts | Bad membrane preparation | Try a new membrane preparation |
| Degraded radioligand | Use a fresh batch of radioligand | |
| Concentration of membrane or radioligand too low | Increase the concentration in your assay | |
| Sticky ligand | You may need a carrier molecule in buffer (BSA or gelatin); high non-specific binding on glassware | |
| Wrong glassware | Some plastic plates, tubes or tips can be responsible for non-specific binding of a radioligand or cold ligand. The compatibility of the counter and experimental lab-ware should be verified priorprior to the experiment. | |
| Shifted affinity | Radioligand is expired | Use a fresh batch of radioligand |
| Cold ligand is degraded | Try a new lot of cold ligand | |
| Sticky ligand | You may need a carrier molecule in buffer (BSA or gelatin); high non-specific binding on glassware | |
| Ligand depletion | Increase assay volume, decrease the quantity of membranes or increase radioligand concentration in competition experiments | |
| High variability | Viscous or non-homogenous membrane preparation | Pass membrane through 1cc syringe with 18.5G needle |
| Sticky ligand | You may need a carrier molecule in buffer (BSA or gelatin); high non-specific binding on glassware | |
| Wrong glassware | Some plastic plates, tubes or tips can be responsible for non-specific binding of a radioligand or cold ligand. The compatibility of the counter and experimental lab-ware should be verified priorprior to the experiment. | |
| Wrong wash buffer | Check your buffer compositions and make sure these have been optimized for your assay | |
| Compound instability | Try using freshly-prepared ligands or compounds. You may also want to switch to a different radioligand. | |
| Manipulation/pipetting problem | We recommend switching tips when you are moving between different samples or concentrations. For sticky ligands, we usually put a new tip and pipet the solution up and down one or two times before transferring. This will help pre-coat the inside of the pipet tip, so that more ligand is transferred when you dispense. |
Citations
- Esbenshade, T.A. et al. Pharmacological and behavioral properties of A-349821, a selective and potent human histamine H3 receptor antagonist. Biochem. Pharmacol 68, 933-945 (2004). Link
- Fujiwara, Y. et al. Mutual regulation of vasopressin- and oxytocin-induced glucagon secretion in V1b vasopressin receptor knockout mice. J Endocrinol 192, 361-369 (2007). Link
- Nourian, Z., Mulvany, M.J., Nielsen, K.B., Pickering, D.S. & Kristensen, T. The antagonistic effect of antipsychotic drugs on a HEK293 cell line stably expressing human alpha1A1-adrenoceptors. Eur. J. Pharmacol 596, 32-40 (2008). Link
- Schepmann, D., Frehland, B., Lehmkuhl, K., Tewes, B. & Wünsch, B. Development of a selective competitive receptor binding assay for the determination of the affinity to NR2B containing NMDA receptors. J Pharm Biomed Anal 53, 603-608 (2010). Link
- Verzijl, D. et al. Noncompetitive Antagonism and Inverse Agonism as Mechanism of Action of Nonpeptidergic Antagonists at Primate and Rodent CXCR3 Chemokine Receptors. Journal of Pharmacology and Experimental Therapeutics 325, 544-555 (2008). Link
Other Revvity ligand-binding technologies
DELFIA fluorescent ligand-binding assays
Custom radiochemicals and cell membranes
Revvity offers custom radiochemicals, cell membranes, and plate barcoding, as well as custom assay development. If you are interested in custom services, please contact us.
Radiosynthesis and Labeling Custom Services
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























