Overview
The table below shows results from a compound interference study. We tested a panel of 24 compounds in a QC assay using our AlphaLISA™ toolbox bead products. The information below can be used for guidance as to what buffer components might interfere with AlphaLISA assays using Anti-Rabbit IgG Alpha Donor beads, and at what concentrations interference might occur. These data are derived from single experiments and should serve more as a guide rather than a precise value, as it is unlikely you will be using the exact same assay design as used to generate the data below. Interference concentrations may vary depending on the assay components. It is possible your assay will tolerate higher or lower concentration than what is shown.
| No effect 10% |
50% loss | Tested up to | % of inhibition at max conc | ||
|---|---|---|---|---|---|
| Glycerol | % | 0.2 | 3.9 | 30% | 91 |
| DMSO | % | 0.5 | 1.8 | 10% | 87 |
| Gelatin | % | >1 | >1 | 1% | 0 |
| CHAPS | % | 0.04 | 0.1 | 3% | 84 |
| Deoxycholate | % | 0.005 | 0.02 | 3% | 94 |
| SDS | % | 0.07 | 0.1 | 3% | 96 |
| Triton X-100 | % | >3 | >3 | 3% | 0 |
| Tween 20 | % | >3 | >3 | 3% | 0 |
| TRIS (pH 8.0) | M | 5.2E-02 | 4.5E-01 | 0.5M | 53 |
| 2-Mercaptoethanol | M | 5.6E-04 | 1.1E-02 | 0.1M | 82 |
| DTT | M | 4.9E-08 | 6.1E-03 | 0.1M | 75 |
| EDTA | M | 4.3E-02 | >0.1 | 0.1M | 38 |
| EGTA | M | 6.6E-03 | >0.1 | 0.1M | 34 |
| IBMX | M | 1.0E-06 | >0.0025 | 0.0025M | 37 |
| Imidazole | M | 1.3E-03 | 7.9E-03 | 0.1M | 99 |
| Mg2+ (MgCl2) | M | >0.1 | >0.1 | 0.1M | 0 |
| Nitroprusside | M | 2.6E-03 | 2.9E-02 | 0.1M | 65 |
| Urea | M | >0.1 | >0.1 | 0.1M | 0 |
| Vanadate | M | 9.8E-03 | 1.1E-02 | 0.1M | 43 |
| Citrate | M | 1.7E-03 | 3.9E-03 | 0.1M | 100 |
| Adenosine (ATP) | M | >0.01 | >0.01 | 0.01M | 0 |
| Glycine | M | 1.9E-06 | >0.01 | 0.01M | 15 |
| NaF | M | >0.01 | >0.01 | 0.01M | 0 |
| S-adenosylmethionine | M | >0.001 | >0.001 | 0.001M | 0 |
| Complete Protease Inhibitor | X | not determined | not determined | 1X | 29 |
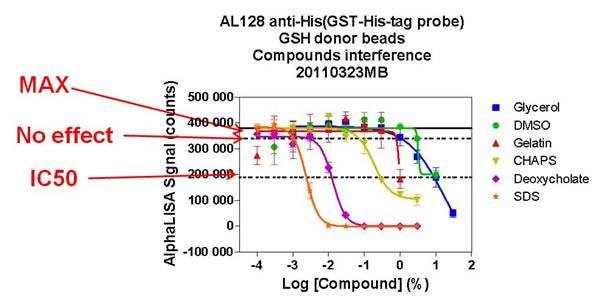
Refer to the figure below for more detail as to what the values in the table above indicate. Please note that the figure is provided as a general example to explain how the values in our interference tables were determined. The table above indicates that an assay testing the effect of different chemicals using Anti-Rabbit IgG Donor beads (with Streptavidin AlphaLISA Acceptor beads) showed little effect on the assay signal at final Triton X-100 concentrations up to 3%. Using 3.9% glycerol resulted in a 50% loss of signal.

Figure: Key for how the values in our interference tables are derived. The “no effect” concentration was obtained by extrapolating the MAX - 10% MAX counts. Refer to the table above for actual interference values for each toolbox bead product.
For research use only. Not for use in diagnostic procedures.




























